Clinical
Pharmacology of SSRI's
- Index |
|
| TABLE 7.1 STEPS Criteria for
Selecting an Antidepressant |
|
Safety
- Therapeutic index
- Drug-drug interactions
Pharmacodynamics
Pharmacokinetics
Tolerability
Efficacy
- Overall
- Unique spectrum of activity
- Rate of response
- Maintenance and prophylaxis
Payment
Simplicity
|
| Adapted from: Preskorn SH. J
Clin Psychiatry. 1994;55(suppl A):6-22, 23-24, 98-100. |
| TABLE 7.2 STEPS Criteria for
Mixed Reuptake and Neuroreceptor Antagonists (eg, Amitriptyline)
Safety |
|
Safety
Therapeutic index: narrow. Serious toxicity can result
from overdose, either acute ingestion or from gradual
accumulation due to slow clearance
Drug-drug interactions:
Pharmacodynamic: prone to cause multiple types of
such interactions due to their multiple mechanisms of
action (Table 6.3)
Pharmacokinetic: can be the target of such interactions
but are not prone to cause them. Due to narrow therapeutic
index, care should be taken when prescribing TCAs to
patients on drugs known to inhibit CYP enzymes (Table
6.10)
Tolerability
Poor due to numerous types of adverse effects mediated
by the blockade of histamine receptors (eg, sedation),
muscarinic acetylcholine receptors (eg, constipation),
and alpha-1adrenergic receptors (eg, orthostatic hypotension)
(Tables 6.2, 6.3, and 6.7)
Efficacy
Overall: highest response rates of any antidepressants.
Clomipramine in particular has been found to be superior
to two SSRIs, citalopram and paroxetine, in hospitalized
patients with clinical depression
Unique spectrum of activity: imipramine has been found
effective in patients who have not benefited from treatment
with the SSRI, sertraline
Rate of response: 2 to 4 weeks
Maintenance of response: established by controlled
studies Payment
Generic versions are available making these antidepressants
the least expensive in terms of acquisition costs. These
savings are likely offset by expenses arising from problems
with their safety and tolerability (Table 7.10)
Simplicity
Must titrate dose due to tolerability problems. Therapeutic
drug monitoring should be done once early in treatment
to guide dose adjustment. Therapeutic ranges have been
established for most of these TCAs. Can be given once
a day
|
| Adapted from: Preskorn SH. J
Clin Psychiatry. 1994;55(suppl A):6-22, 23-24, 98-100. |
| TABLE 7.3 STEPS Criteria for
Norepinephrine Selective Reuptake Inhibitors (eg, Desipramine) |
|
Safety
Therapeutic index: narrow. The only NSRIs available
at present in the United States are secondary amine
TCAs which have the same toxicity problems as tertiary
amine TCAs (eg, amitriptyline). However, this safety
problem is due to inhibition of fast sodium channels
as opposed to norepinephrine reuptake inhibition. In
fact, there are investigational, nontricyclic NSRIs
(eg, reboxetine) which do not have a narrow therapeutic
index in terms of cardiotoxicity
Drug-drug interactions:
Pharmacodynamic: limited at therapeutic concentrations
to those produced by norepinephrine potentiation
Pharmacokinetic: can be target of such interactions
but not prone to cause them. Due to narrow therapeutic
index, care should be taken when prescribing with drugs
known to inhibit CYP enzymes (Table 6.10)
Tolerability
Generally good. Adverse effects mediated by norepinephrine
potentiation (Tables 6.2, 6.3, and 6.6)
Efficacy
Overall: comparable to tertiary amine TCAs
Unique spectrum of activity: have been found in several
studies to be effective in approximately 50% of patients
who have not benefited from treatment with a variety
of SSRIs Rate of response: 2 to 4 weeks
Maintenance of response: established by controlled
studies Payment
Generic versions are available but cost almost as
much as newer antidepressants (Table 7.10)
Simplicity
Can start on an effective dose immediately. Therapeutic
drug monitoring should be done once early in treatment
to guide dose adjustment. Therapeutic ranges have been
established for most of these TCAs. Can be given once
a day
|
| Abbreviations: NSRI, norepinephrine
selective reuptake inhibitor; TCA, tricyclic antidepressant;
CYP, cytochrome P450 enzyme; SSRI, serotonin selective
reuptake inhibitor. |
| TABLE 7.4 STEPS Criteria for
Serotonin Selective Reuptake Inhibitors (eg, Sertraline) |
|
Safety
Therapeutic index: wide. No serious systemic toxicity
demonstrated, even after substantial overdose
Drug-drug interactions:
Pharmacodynamic: serotonin syndrome may occur when
used with other serotonin agonists. Can potentiate dopamine
antagonists in terms of extrapyramidal effects (eg,
motor restlessness)
Pharmacokinetic: considerable differences exist among
SSRIs in terms of their potential for decreasing the
rate of oxidative metabolism of a variety of drugs by
inhibiting CYP enzymes: fluoxetine, fluvoxamine > paroxetine
> citalopram, sertraline (Table 6.10). No known clinically
significant effect of other drugs on the clearance of
SSRIs
Tolerability
Good. Nausea and loose stools can occur early and
are dose dependent but tolerance typically develops.
Sexual dysfunction (eg, anorgasmia) can occur in approximately
30% of patients (Tables 6.2, 6.3, and 6.6) Efficacy
Overall: equivalent to TCAs in outpatients
Unique spectrum of activity: can work in TCAs nonresponders
Rate of response: 2 to 4 weeks
Maintenance of response: evidence from controlled
studies Payment
Brand name only available; see discussion (Table 7.10)
Simplicity
Ease of administration: fluoxetine, paroxetine, and
sertraline can be started at an effective dose immediately.
Nonlinear pharmacokinetics of paroxetine likely contribute
to an increased risk of experiencing an SRI discontinuation
syndrome after abrupt cessation. The long half-life
of fluoxetine and active metabolite, norfluoxetine,
means time to maximum effect and time to washout can
take up to 2 months
|
| Abbreviations: SSRI, serotonin
selective reuptake inhibitor; CYP, cytochrome P450 enzyme;
TCA, tricyclic antidepressant; SRI, serotonin reuptake
inhibitor. |
| TABLE 7.5 STEPS Criteria for
Serotonin and Norepinephrine Reuptake Inhibitors (eg,
Venlafaxine) |
|
Safety
Therapeutic index: wide. No serious systemic toxicity
demonstrated. Dose-dependent hypertension going from
1% above placebo at doses £ 100 mg/day to 11% above
placebo at doses ³ 300 mg/day. That fact is consistent
with NE uptake inhibition occurring at higher doses
Drug-drug interaction:
Pharmacodynamic: same as SSRIs at low doses. NE-mediated
interactions possible at higher doses
Pharmacokinetic: has profile comparable to citalopram
and sertraline in terms of no to minimal effects on
most CYP enzymes (Table 6.10)
Tolerability
Comparable to SSRIs at low dose; NE-mediated adverse
effects at higher doses (Tables 6.2, 6.3, and 6.7)
Efficacy
Overall: equivalent to TCAs. High-dose venlafaxine
was superior to fluoxetine in double-blind study
Unique spectrum of activity: possible efficacy in
cases not responsive to TCAs or SSRIs
Rate of response is a function of dose: 2 to 4 weeks
at doses £ 150 mg/day and 4 to 7 days at doses of ³
300 mg/day
Maintenance of response: evidence from controlled
studies
Payment
Brand name only available; see discussion (Table 7.10)
Simplicity
Ease of administration: can be started at an effective
dosage (75 mg/day); once daily dosing with sustained-release
formulation. Dose titration is not necessary for many
patients but increasing the dose is a reasonable strategy
if starting dose is ineffective
|
| Abbreviations: NE, norepinephrine;
SSRI, serotonin selective reuptake inhibitor; CYP, cytochrome
P450 enzyme; TCA, tricyclic antidepressant. |
| TABLE 7.6 STEPS Criteria for
Serotonin-2A Receptor Antagonist and Weak Serotonin Reuptake
Inhibitors (eg, Nefazodone) |
|
Safety
Therapeutic index: wide. No serious systemic toxicity
due to acute overdose
Drug-drug interactions:
Pharmacodynamic: can interact with other agents that
decrease arousal or impair cognitive performance. Can
interact with adrenergic agents affecting blood pressure
regulation. Complex interactions with other serotonin-active
agents
Pharmacokinetic: substantially inhibits CYP 3A which
is responsible for 50% of known oxidative drug metabolism,
including its own metabolism (ie, nonlinear pharmacokinetics
or autoinhibition)
Tolerability
Dizziness, drowsiness, and confusion are dose-dependent
adverse effects; hence the need for dose titration (Tables
6.2, 6.3, and 6.7)
Efficacy
Overall: equivalent to TCAs in outpatients
Unique spectrum of activity: none demonstrated
Rate of response: 2 to 4 weeks
Maintenance of response: evidence from controlled
studies Payment
Generic version of trazodone available. Brand name
only for nefazodone. The latter has clinically important
pharmacologic advantages over the former (Table 7.10)
Simplicity
Ease of administration: requires dose titration and
divided daily dosing for optimal antidepressant effect
Safety
Therapeutic index: wide. No serious systemic toxicity
due to acute overdose
Drug-drug interactions:
Pharmacodynamic: can interact with other agents that
decrease arousal or impair cognitive performance. Can
interact with adrenergic agents affecting blood pressure
regulation. Complex interactions with other serotonin-active
agents
Pharmacokinetic: substantially inhibits CYP 3A which
is responsible for 50% of known oxidative drug metabolism,
including its own metabolism (ie, nonlinear pharmacokinetics
or autoinhibition)
Tolerability
Dizziness, drowsiness, and confusion are dose-dependent
adverse effects; hence the need for dose titration (Tables
6.2, 6.3, and 6.7)
Efficacy
Overall: equivalent to TCAs in outpatients
Unique spectrum of activity: none demonstrated
Rate of response: 2 to 4 weeks
Maintenance of response: evidence from controlled
studies Payment
Generic version of trazodone available. Brand name
only for nefazodone. The latter has clinically important
pharmacologic advantages over the former (Table 7.10)
Simplicity
Ease of administration: requires dose titration and
divided daily dosing for optimal antidepressant effect
|
| Abbreviations: CYP,
cytochrome P450 enzyme; TCA, tricyclic antidepressant. |
| TABLE 7.7 STEPS Criteria for
Serotonin (5-HT2A and 5-HT2C) and Norepinephrine (a-2)
Receptor Antagonists (eg, Mirtazapine) |
|
Safety
Therapeutic index: wide in terms of acute drug overdose.
There were three cases of agranulocytosis out of approximately
3000 patients in clinical trial program. That number
was too small to establish with confidence the actual
incidence or even whether there was a causal relation.
Postmarketing experience has not indicated that toxicity
is a major concern. Nevertheless, a white count should
be obtained if a patient presents with signs of fever
or infection
Drug-drug interaction:
Pharmacodynamic: can cause multiple types of such
interactions due to multiple mechanisms of action (Table
6.3)
Pharmacokinetic: unlikely to either be a victim or
a cause of such interactions based on in vitro studies,
but that should be confirmed by in vivo studies
Tolerability
Primary problems are sedation due to histamine receptor
blockade and weight gain due to 5-HT2C receptor blockade.
These can be treatment limiting (Tables 6.2, 6.3, and
6.7). Dose titration may not help
Efficacy
Overall: good; was superior to fluoxetine in double-blind
study of hospitalized patients
Unique spectrum of activity: worked in 54% of patients
who had not benefited from treatment with amitriptyline.
Open label studies have reported effectiveness in patients
who had not benefited from SSRIs
Rate of response: 2 to 4 weeks
Maintenance of response: data limited to noncontrolled
open label extension studies
Payment
Brand name only available; see discussion (Table 7.10)
Simplicity
Can start on an effective dose immediately. Sedation
can be a problem, but starting with a lower dose may
only decrease efficacy without improving tolerability,
because mirtazapine is a more potent histamine receptor
blocker than it is a serotonin and adrenergic receptor
blocker. No rigorous data on whether higher doses increase
either efficacy or tolerability
|
| TABLE 7.8 STEPS Criteria for
Dopamine and Norepinephrine Reuptake Inhibitors (eg, Bupropion) |
|
Safety
Therapeutic index: narrow. The dose needed for efficacy
(300-450 mg/day) is only 2 to 3 times less than the
dose that causes seizures in 2% of patients. That is
the reason the maximum recommended daily dose is 450
mg/day. Seizures can also occur following an acute overdose
but are generally managed easily in a medical setting
Drug-drug interaction:
Pharmacodynamic: should potentiate and reduce the
effects of other dopamine and norepinephrine agonists
and antagonists, respectively
Pharmacokinetic: can be affected by fluoxetine and
probably others in a clinically significant way. Due
to its narrow therapeutic index, care should be taken
when prescribing bupropion to patients on drugs known
to inhibit CYP enzymes. Case report data suggest that
it can substantially inhibit CYP 2D6
Tolerability
Good. Does not cause sexual dysfunction seen with
antidepressants which are serotonin reuptake inhibitors
(Tables 6.2, 6.3, and 6.7)
Efficacy
Overall: probably less than TCAs Unique spectrum
of activity: can work in TCA nonresponders
Rate of response: 2 to 4 weeks
Maintenance of response: not demonstrated in formal
studies
Payment
Brand name only available; see discussion (Table 7.10)
Simplicity
Ease of administration: requires divided daily dosing
for antidepressant effect and dose titration to achieve
efficacy and minimize seizure risk
|
| Abbreviations: CYP, cytochrome
P450 enzyme; TCA, tricyclic antidepressant. |
| TABLE 7.9 STEPS Criteria for
Monoamine Oxidase Inhibitors (eg, Tranylcypromine) |
|
Safety
Therapeutic index: narrow. Serious toxicity can result
from acute overdose
Drug-drug interaction:
Pharmacodynamic: hypertensive crisis can result from
coadministration with tyramine and sympathomimetic agents.
Serotonin syndrome can result from coadministration
with serotonin agonists (eg, serotonin uptake inhibitors)
(Table 6.2)
Pharmacokinetic: inhibits the oxidative enzyme, MAOI,
but effects on CYP enzymes have not been studied. Not
known to be affected by other drugs in a clinically
significant way
Tolerability
Generally good, especially if kept to effective minimum
dose. Ironically, main tolerability problem is hypotension
(Table 6.3)
Efficacy
Overall: Probably less than TCAs
Unique spectrum of activity: can work in TCA nonresponders
Rate of response: 2 to 4 weeks
Maintenance of response: not adequately tested
Payment
Generic versions are available making these antidepressants
the least expensive in terms of acquisition costs (Table
7.10). These savings are likely offset by expenses arising
from problems with their safety and tolerability
Simplicity
Ease of administration: typically administered in
divided daily doses. Dose titration recommended to optimize
efficacy and minimize hypotension
|
| Abbreviations: MAOI, monoamine
oxidase inhibitor; CYP, cytochrome P450 enzyme; TCA, tricyclic
antidepressant. |
| TABLE 7.10 Average
Wholesale Price of Representative Doses of Antidepressants* |
| Generic/Trade
Drug Name |
Strength
(mg) |
Price
($/100) |
| Generic
|
Trade |
| Tertiary Amine
Tricyclic Antidepressants |
|
| Amitriptyline/Elavill |
25 |
10 |
40 |
| 50 |
12 |
72 |
| 100 |
18 |
125 |
| Doxepin/Sinequan |
25 |
14 |
47 |
| 50 |
19 |
65 |
| 100 |
40 |
119 |
| Imipramine/Tofranil |
25 |
6 |
47 |
| 50 |
9 |
80 |
| Selective Norepinephrine
Reuptake Inhibitors |
|
| Desipramine/Norpramin |
25 |
25 |
65 |
| 50 |
50 |
122 |
| 100 |
100 |
204 |
| Nortriptyline/Pamelor |
25 |
80 |
100 |
| 50 |
150 |
190 |
| Serotonin Selective
Reuptake Inhibitors |
|
| Citalopram/Celexa |
20 |
NA |
193 |
| 40 |
NA |
202 |
| Fluoxetine/Prozac |
10 |
NA |
230 |
| 20 |
NA |
240 |
| Fluvoxamine/Luvox |
50 |
NA |
206 |
| 100 |
NA |
212 |
| Paroxetine/Paxil |
10 |
NA |
190 |
| 20 |
NA |
200 |
| 30 |
NA |
210 |
| 40 |
NA |
222 |
| Sertraline/Zoloft |
50 |
NA |
180 |
| 100 |
NA |
200 |
| Serotonin and
Norepineprhine Reuptake Inhibitors |
|
| Venlafaxine-IR/Effexor
IR |
37.5 |
NA |
105 |
| 75 |
NA |
115 |
| 100 |
NA |
122 |
| Venlafaxine-XR/Effexor
XR |
37.5 |
NA |
194 |
| 75 |
NA |
217 |
| 150 |
NA |
237 |
| Serotonin 2A Antagonists |
|
| Nefazodone/Serzone |
100,
150, 200, 250 |
NA |
93 |
| Trazodone/Desyrel |
50 |
30 |
150 |
| 100 |
40 |
250 |
| 300 |
NA |
390 |
Serotonin (5-HT2A
and 2C) and Adrenergic (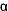 -2)
Antagonists -2)
Antagonists |
|
| Mirtazapine/Remeron |
15 |
NA |
195 |
| 30 |
NA |
202 |
| Dopamine and Norepinephrine
Reuptake Inhibitors |
|
| Bupropion-IR/Wellbutrin
IR |
75 |
NA |
60 |
| 100 |
NA |
83 |
| Bupropion-SR/Wellbutrin
SR |
100 |
NA |
115 |
| 150 |
NA |
117 |
| Monoamine Oxidase
Inhibitors |
|
| Phenelzine/Nardil |
15 |
NA |
42 |
| Tranylcypromine/Parnate |
10 |
NA |
50 |
| Abbreviations: NA,
none available; IR, immediate release; SR, sustained release;
XR, extended release. |
| * Information taken
from 1998 Drug Topics Red Book and rounded to the
nearest dollar. Recommended dosing is twice or three
times a day, whereas other drugs in this chart are given
once a day. That difference will affect the daily cost
of treatment (ie, how long 100 pills will last). |
| TABLE 7.11 Summary
of Package Insert Dosing Guidelines |
| Generic/Trade
Drug Name |
Recommended Dose
Start/ Max (mg/day) |
Dosage Guidelines
for Specific Patients |
| Children |
Adolescents |
Elderly |
Hepatic* |
Renal* |
| Mixed Reuptake
Inhibitors and Neuroreceptor Blockersa,b |
|
| Amitriptyline/Elavil |
75/300c |
 |
NA |
 |
 |
 |
| Amoxapine/Ascendin |
100/600c,d |
NR |
NA |
 |
 |
 |
| Clomipramine/Anafranile |
25/250c,f |
NA |
NA |
 |
 |
 |
| Doxepin/Sinequan |
75/300c |
NA |
NA |
NA |
 |
 |
| Imipramine/Tofranil |
75/300cc |
NA |
 |
 |
 |
 |
| Norepinephrine
Selective Reuptake Inhibitorsa,b |
|
| Desipramine/Norpramin |
100/300c |
 |
NA |
 |
 |
 |
| Maprotiline/Ludiomil |
75/225c,f |
NA |
NA |
 |
 |
 |
| Nortriptyline/Pamelor |
50/150c |
NA |
NA |
 |
 |
 |
| Serotonin Selective
Reuptake Inhibitors |
|
| Citalopram/Celexa |
20/60 |
NA |
NA |
 |
 |
 |
| Fluoxetine/Prozac |
20/80 |
NA |
NA |
 |
 |
 |
| Fluvoxamine/Luvoxe |
50/300g |
NR |
NA |
 |
 |
 |
| Paroxetine/Paxil |
20/50 |
NA |
NA |
 |
 |
 |
| Sertraline/Zoloft |
50/200 |
 |
Same |
Same |
 |
 |
| Serotonin and
Norepinephrine Reuptake Inhibitors |
|
| Venlafaxine-IR/Effexor IR |
75g,h/375g |
NA |
NA |
NA |
 |
 |
| Venlafaxine-XR/Effexor XR |
75h/375 |
NA |
NA |
NA |
 |
 |
| Serotonin (5-HT2A)
Receptor Blockers and Weak Serotonin Uptake Inhibitors |
|
| Nefazodone/Serzone |
200g/600g |
NA |
NA |
 |
 |
 |
| Trazodone/Dyserel |
150g/600g |
NA |
NA |
 |
 |
 |
| Serotonin (5-HT2A
and 5-HT2C) and Norepinephrine Receptor Blockers |
|
| Mirtazapine/Remeron |
15/45 |
NA |
NA |
 |
 |
 |
| Dopamine and Norepinephrine
Reuptake Inhibitors |
|
| Bupropion-IR/Wellbutrin IR |
200g/450c,f,i |
NA |
NA |
 |
 |
 |
| Bupropion-SR/Wellbutrin SR |
150/400c,f,i |
NA |
NA |
 |
 |
 |
| Monoamine Oxidase
Inhibitors |
|
| Phenelzine/Nardil |
45g/90g |
NA |
NA |
 |
 |
 |
| Tranylcypromine/Parnate |
30g/60g |
NA |
NA |
 |
 |
 |
| Abbreviations: NA,
not available; NR, not recommended; IR, immediate release;
XR, extended release; SR, sustained release. |
| * Impairment. |
- Starting dose may be given either as a once-a-day
dose or on a divided schedule. Once an effective and
tolerated dose has been established, it may be given
on a once-a-day basis, but a divided dose may still
be more prudent with a higher total dose and in patients
who are elderly or debilitated. The maximum once-a-day
dose of doxepin is 150 mg.
- Usual dose may be given either as a once-a-day
dose or on a divided schedule.
- Therapeutic drug monitoring has been either demonstrated
to increase the safe and efficacious use of this drug
or theoretically should; demonstrated for amitriptyline,
clomipramine, desipramine, imipramine, and nortriptyline.
Theoretical for the rest, but has not been adequately
studied.
- Doses should exceed 400 mg/day only in hospitalized
patients who do not have a history of seizures and
who have not benefited from an adequate trial of 400
mg/day.
- Not formally labeled by the FDA for the treatment
of clinical depression but rather for obsessive-compulsive
disorder; labeled for use as an antidepressant in
other countries.
- Maximum daily dose should not be exceeded due to
an increased risk of seizures.
- Dose should be given on a divided schedule (bid
or tid).
- For some patients, it may be desirable to start
at half the dose for 4 to 7 days to improve tolerance,
particularly in terms of nausea.
- It is particularly important to administer in a
manner most likely to minimize the risk of seizures.
Dose increases should not exceed 100 mg/day in a 3-day
period. Cautious dose titration can also minimize
agitation, motor restlessness, and insomnia. Time
between doses should be at least 4 hours for 100 mg
IR doses, 6 hours for 150 mg IR doses, and 8 hours
for SR doses. Increases above 300 mg/day should only
be done in patients with no clinical effects after
several weeks of treatment at 300 mg/day. Bupropion
should be discontinued in patients who do not experience
an adequate response after an adequate period on maximum
recommended daily dose. Dosing in the elderly, the
debilitated, and patients with hepatic and/or renal
impairment has not been adequately studied so increased
caution may be prudent.
|
|
Additional comments on dose titration: The package
inserts for the following drugs indicate that they can
be started at a dose which is usually effective to treat
clinical depression: fluoxetine, mirtazapine, paroxetine,
tranylcypromine, sertraline, venlafaxine. The following
comments apply about the use of higher doses with these
antidepressants. For fluoxetine, paroxetine, and
sertraline: although fixed-dose studies in patients
with clinical depression found no advantage on average
to higher doses, an increase may be considered after
several weeks on the starting dose if no clinical improvement
has been observed. For mirtazapine, dose escalation
should not be made at intervals of less than 1 to 2
weeks to adequately evaluate therapeutic response to
a given dose. For tranylcypromine, improvement
can be seen between 48 hours and 2 weeks of starting
therapy; if not, dose increases in 10 mg/day increments
may be made at intervals of 1 to 3 weeks.
The package inserts for the following drugs recommend
starting at a lower than usually effective dose and
titrate up to a dose which is usually effective to treat
clinical depression in order to minimize tolerability
or safety problems: amitriptyline, amoxapine, bupropion,
citalopram, clomipramine, fluvoxamine, doxepin, imipramine,
nefazodone, phenelzine, trazodone, and trimipramine.
The following are additional comments about dose titration
with these antidepressants: For the tricyclic antidepressants,
the dose should be gradually increased during the first
2 weeks based on therapeutic drug monitoring and clinical
assessment of efficacy and tolerability. For fluvoxamine,
a lower than usually effective starting dose is recommended
to improve tolerability. The dose should be increased
every 4 to 7 days as tolerated until maximum therapeutic
benefit is achieved. For citalopram, the starting
dose is 20 mg/day with the recommendation to generally
increase to 40 mg/day. While doses above 40 mg/day are
not ordinarily recommended, some patients may require
a dose of 60 mg/day. For nefazodone, a lower
than usually effective starting dose is recommended
to improve tolerability. Dose titration should occur
in increments of 100 to 200 mg/day as determined by
tolerability and the need for further clinical improvement.
These incremental advances should be done using divided
doses and at intervals of at least 1 week. It may be
advisable to titrate up more slowly in elderly and debilitated
patients. For phenelzine, a lower than usually
effective starting dose is recommended to improve tolerability.
Its dose should be increased to at least 60 mg/day at
a fairly rapid pace consistent with good tolerability.
For trazodone, the same comments apply as for nefazodone
when trazodone is used as an antidepressant; however,
it is now mainly used as a nonhabit-forming sedative
given as a single bedtime dose of 50 to 200 mg as needed
for sleep.
|
| TABLE
8.1 Percentage of New Prescriptions Written for Specific
Antidepressants During October, 1998 |
| Antidepressant |
Prescription (%) |
| Mixed
Uptake and Neuroreceptor Blockers (17.9) |
| Amitriptyline |
12.2 |
| Doxepin |
2.8 |
| Imipramine |
2.9 |
| Norepinephrine
Selective Reuptake Inhibitors (4.4) |
| Desipramine |
0.8 |
| Nortriptyline |
3.6 |
| Serotonin
Selective Reuptake Inhibitors (50.5) |
| Citalopram |
1.1 |
| Fluoxetine |
17.5 |
| Fluvoxamine |
1.2 |
| Paroxetine |
14.5 |
| Sertraline |
16.2 |
| Serotonin
and Norepinephrine Reuptake Inhibitors (5.0) |
| Venlafaxine-XR |
3.1 |
| Venlafaxine-IR |
1.9 |
| Serotonin-2A
Blockers* (12.0) |
| Nefazodone |
3.5 |
| Trazodone |
8.5 |
| Specific
Serotonin and Adrenergic Receptor Blocker
(1.9) |
| Mirtazapine |
1.9 |
| Dopamine
and Norepinephrine Reuptake Inhibitor (7.9) |
| Bupropion-SR |
6.3 |
| Bupropion-IR |
1.6 |
| Monoamine
Oxidase Inhibitors (< 0.1) |
| Abbreviations: TCA,
tricyclic antidepressant; XR, extended release; IR, immediate
release; SR, sustained release. |
| * Also weakly inhibits
the serotonin uptake pump. Most potent action is histamine
receptor blockade. |
| TABLE
8.2 Indications Formally Labeled By the FDA for Specific
Serotonin Selective Reuptake Inhibitors* |
| SSRI |
Bulimia Nervosa |
Depression |
OCD |
Panic Disorder |
PTSD |
| Citalopram |
No |
Yes |
No |
No |
No |
| Fluvoxamine |
No |
No |
Yes |
No |
No |
| Fluoxetine |
Yes |
Yes |
Yes |
No |
No |
| Paroxetine |
No |
Yes |
Yes |
Yes |
No |
| Sertraline |
No |
Yes |
Yes |
Yes |
Yes |
| Abbreviations: FDA,
Food and Drug Administration; SSRI, serotonin selective
reuptake inhibitor; OCD, obsessive-compulsive disorder;
PTSD, posttraumatic stress disorder. |
| * All other antidepressants
are solely labeled for the treatment of depression with
the exception of bupropion which is marketed under the
brand name Zyban as an aid for smoking cessation. Venlafaxine
is indicated for the treatment of patients with generalized
anxiety disorder. Paroxetine is indicated for the treatment
of patients with social anxiety disorder. Trials with
sertraline are being reviewed by the FDA for the possible
labeling as indicated for the treatment of patients with
double depression and dysthymia. |
|

