| Preskorn.com | Printed from: http://www.preskorn.com/columns/0209.html |
| Clinical Pharmacology Case Conference: A Suicide Attempt? |
|
|
|
SHELDON H. PRESKORN, MD |
|
Journal of Psychiatric Practice, September 2002, 306-310 |
This column is another in the series illustrating basic clinical pharmacology principles. The following two related equations are fundamental to these case presentations:
Effect = affinity for x drug x biological
site of action level variance
(Equation 1)
Drug concentration = dosing rate/clearance
(Equation 2)
As in past columns in this series, the case will be presented followed by questions for the reader to consider before reading the discussion.
Case Report
The patient was a 40-year-old married woman with major depression and an addiction to pain medications. She was being treated by a primary care physician. For more than the past month, she had been on the following medication regimen:
| amitriptyline ranitidine ketoprofen cisapride hydrocodone* acetaminophen† isomethetpine‡ dichloralphenazone‡ | 100 mg/day 300 mg/day 100 mg/day 40 mg/day 5 mg (up to 20 mg in 12 hrs) 325-500 mg (up to 3625 mg in 12 hrs) 65 mg (up to 325 mg in 12 hrs) 100 mg (up to 500 mg in 12 hrs) |
| * Given as the combination product, Lortabs, which contains both hydrocodone and 500 mg of acetaminophen † Present in both combination products, Lortabs and Midrin. Not clear that the doses of those combination products were adjusted to account for the presence of acetaminophen in both products. ‡ Given as the combination product, Midrin, which contains isomethetpine, dichloralphenazone, and 325 mg of acetaminophen. | |
Within the preceding few weeks, fluoxetine had been added to the patient's regimen and the dose increased to 40 mg/day.
On December 16, the patient went to a Christmas party with her husband, where she had one "Long Island Iced Tea." She and her husband returned home and, at approximately 03:00 am on December 17, she took her amitriptyline and went to bed.
At 04:30 am, her labored breathing awakened her husband. He could not rouse her and called an ambulance, which transported the patient to the hospital. Her blood alcohol level drawn in the emergency room at 05:50 am was 130 mg/100 mL. An electrocardiogram (ECG) revealed a supraventricular tachycardia and a prolonged QRS interval. The patient remained obtunded and was transferred to an intensive care unit.
Her tricyclic (amitriptyline + nortriptyline) plasma level at 13:50 on 12/17 was 2040 ng/mL. Subsequent tricyclic levels were 962 ng/mL at 21:55 on 12/17, 641 ng/mL at 13:05 on 12/19, and 495 ng/mL at 12:50 on 12/21. Her fluoxetine and norfluoxetine levels measured at 13:50 on 12/17 were 130 and 204 ng/mL, respectively.
When the patient awoke, a psychiatric consultation was obtained. The patient reported that her depressive illness had begun at age 14 and that, when she was 21, she had attempted suicide by "taking every pill in the house." The patient denied any other suicide attempts and specifically denied taking an overdose on 12/17.
Before proceeding to the discussion of this case, consider the following two questions: first, based on the information given above, do you think this patient took an intentional overdose on 12/17? Second, why did this patient have such a rapid decline in her tricyclic plasma level over the first day, followed by a much slower decline over the next several days?
Did This Patient Intentionally Take an Overdose?
This patient clearly experienced a tricyclic antidepressant overdose as witnessed by her obtundation, her ECG findings, and her elevated tricyclic plasma levels.1 The threshold level for toxicity with tricyclic antidepressants is 450 ng/mL.2,3 The likelihood and severity of clinically significant toxicity increase the more the tricyclic level exceeds this threshold value. The toxicity of tricyclic antidepressants includes adverse effects on the brain (e.g., coma) and the heart (e.g., delayed intracardiac conduction).1 This patient's tricyclic plasma levels were well above the toxic threshold and she had documented adverse effects on both brain and heart and was at risk for sudden death because of the magnitude of this overdose.
While these facts document that this patient did experience a tricyclic overdose, the question is whether this overdose was the result of an intentional overdose by the patient (i.e., a suicide attempt) or an unintentional overdose resulting from a pharmacokinetic drug-drug interaction.
This case provides a clinical example of the issues discussed in the previous column, which reviewed 13 deaths following presumed intentional overdoses of fluvoxamine along with other medications.4 Several points were made in that column. One was that, in all 13 cases, patients co-ingested at least one medication whose bioavailability would have been increased and/or whose clearance would have been delayed by fluvoxamine. The increased bioavailability and/or the delayed clearance would have amplified the toxic effects of that co-ingested drug. In 4 of the 13 cases, the co-ingested drug was a tricyclic antidepressant. Of course, ingesting sufficiently large amounts of a tricyclic antidepressant alone can be fatal, due to the narrow therapeutic index of this class of antidepressants.5 Given the data available in those 4 cases, it was not possible to determine to what degree, if any, the co-ingestion of fluvoxamine contributed to the death of the patients.
Another point made in the previous column was that suicide is an obvious consideration and ready explanation for the unexpected death of a patient who is being treated for major depression (i.e., who is taking an antidepressant), especially when no anatomical cause of death (e.g., occlusion of a major coronary artery) is apparent from the autopsy and high levels of a potentially toxic drug (e.g., a tricyclic antidepressant) are found in post-mortem blood or tissue samples. Those facts certainly point to an acute overdose, but they do not help one distinguish between a patient-initiated intentional overdose or a prescriber-initiated unintentional overdose resulting from a pharmacokinetic drug-drug interaction. In the latter case, the overdose can occur because the prescriber has not considered the possibility that one co-prescribed drug will delay the clearance of another co-prescribed drug to such an extent that toxic levels will result. The data available on the 13 cases of overdose deaths involving fluvoxamine presented in the previous column did not permit definite determination of the type of overdoses involved. However, in this case, it is possible to address that question because the patient did not die, so that it was possible to obtain repeated measurements of drug clearance after the overdose.
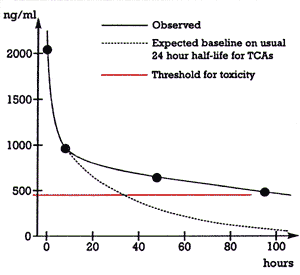
| Figure 1. Total amitriptyline + nortriptyline levels versus time | |
 |
Figure 1 shows the time course for the clearance of the patient's tricyclic (amitriptyline + nortriptyline) level. There was an initial rapid drop from 2040 ng/mL at 13:50 to 962 ng/mL at 21:55 on 12/17 (i.e., a period of 8 hours). However, the clearance became markedly slower after that, falling from 962 ng/mL at 21:55 on 12/17 to 495 ng/mL at 12:50 on 12/21 (i.e., a period of 87 hours).
Figure 1 also shows how the patient's plasma tricyclic levels would have fallen off after 21:55 on 12/17 if the half-life of the drug had been the usual 24 hours in this patient.6 In that case, her plasma tricyclic level would have fallen below the toxicity threshold of 450 ng/mL after approximately 30 hours, and her level would have been approximately 80 ng/mL by 87 hours. Instead, this patient's levels were still above the toxic threshold at 87 hours and approximately six times higher than would have been expected given usual clearance. Figure 1 thus indicates that this patient's clearance of amitriptyline was much slower than would usually be expected. The clearance of amitriptyline and nortriptyline is mediated principally by the drug metabolizing enzyme, cytochrome P450 (CYP) 2D6.7 Conceivably, this patient may have been genetically deficient in this enzyme, which could have resulted in her slow clearance of amitriptyline. This patient was Caucasian and approximately 7% of Caucasians are genetically deficient in this drug metabolizing enzyme.8 Parenthetically, such a genetic deficiency would fall under variable 3 (i.e., biological variance among patients) in equation 1 above.
However, in this case there is another more compelling explanation. This patient was being treated with fluoxetine and had a combined level of fluoxetine + norfluoxetine of 334 ng/mL. At these levels, fluoxetine and its active metabolite will substantially inhibit CYP 2D6 in all normal CYP 2D6 metabolizers. In fact, 40 mg/day of fluoxetine will convert 95% of normal CYP 2D6 metabolizers into phenocopies of genetic deficiency.9 Thus, fluoxetine-induced inhibition of CYP 2D6 was an acquired biological variance (i.e., variable 3 in equation 1) in this patient and shifted her dose-response relationship, putting her at risk for a toxic reaction. By reducing her ability to clear amitriptyline and nortriptyline (Equation 2), the addition of fluoxetine was comparable to substantially increasing her dose of amitriptyline. In this case, the magnitude of the decrease in her clearance (at least 80% on average) was sufficient to cause a potentially fatal, unintentional overdose. Amitriptyline is also N-demethylated by CYP 2C19.7 Fluoxetine at 40 mg/day also converts 80% of normal CYP 2C19 metabolizers to phenocopies of genetic deficiency of this CYP enzyme.10
The inhibition of these two CYP enzymes by fluoxetine is consistent with the slow fall in amitriptyline + nortriptyline levels observed in this patient after 21:55 on 12/17, but it does not explain the rapid fall in these levels between 13:50 and 21:55 on 12/17 (i.e., from 2040 to 962 ng/mL). Such a rapid fall is generally consistent with an acute overdose, suggesting that an acute overdose was superimposed on the chronic overdose caused by the co-administration of fluoxetine (40 mg/day) and amitriptyline (100 mg/day).
Did This Patient Take a Smaller Acute Overdose on 12/17?
The answer is most likely yes, but again, it was likely unintentional and resulted from another drug-drug interaction. Recall that the patient had one "Long Island Iced Tea" at the Christmas party before she returned home and took her usual bedtime dose of amitriptyline. A "Long Island Iced Tea" contains a lot of alcohol, as witnessed by the fact that this patient had a blood alcohol level of 130 mg/100 mL almost 3 hours after she went to bed. Thus, this patient took her evening dose of amitriptyline in the presence of a substantial amount of alcohol, which can interfere with the first pass metabolism of amitriptyline and cause a 2-fold increase in the amount reaching the systemic circulation.11,12
The available data admittedly do not absolutely rule out the possibility that the patient took more amitriptyline than she should have on 12/17. Patients intoxicated to the degree that this patient was have been reported to take more medication than prescribed because they are too cognitively impaired to remember taking their medication. The point of this column is that an intentional overdose does not have to be invoked to explain what happened to this patient. Moreover, an acute overdose does not explain the slow clearance observed in this case.
Instead, the most plausible explanation for all of the facts in this case is that the patient's toxic levels of amitriptyline + nortriptyline resulted from two separate drug-drug interactions that were superimposed on each other. The first was the interaction of fluoxetine and amitriptyline, which led to the chronic accumulation of levels of amitriptyline + nortriptyline above the minimal threshold for toxicity (i.e., 450 ng/mL). In this case, the patient's level of amitriptyline + nortriptyline 12 hours post dose was likely above 900 ng/mL. That level had gradually accumulated over the interval she had been taking fluoxetine in addition to her amitriptyline. Since her fluoxetine + norfluoxetine levels were still accumulating due to the long half-lives of these agents, her amitriptyline + nortriptyline levels would likely have continued to increase over the next several weeks, and that alone could have caused a clinically detectable toxic event.13 As it was, the level of approximately 900 ng/mL was not sufficiently high in this patient to cause clinically apparent toxicity. However, she did experience serious and potentially life-threatening toxicity as a result of a sudden increase in levels due to the interaction of alcohol and amitriptyline (i.e., increased bioavailability of amitriptyline) superimposed on the chronically elevated levels that were already present due to the interaction of fluoxetine and amitriptyline.
Parenthetically, the 900 ng/mL level can be sufficient to cause serious toxicity in some individuals. This again illustrates the role of biological variance (i.e., variable 3 in equation 1 above), which can shift the dose-response curve in patients. Some of the biological variables that can increase the sensitivity of individuals to the toxic effects of tricyclic antidepressants include age and underlying brain and heart disease.2,14
How the Effects of Drug-Drug Interactions Can Be Seen But the Cause Missed
Had this 40-year-old woman died suddenly en route to the hospital or shortly after her arrival, data on antemortem plasma drug levels would not have been obtained. In that case, the patient would likely have been signed out as death due to suicide by intentional drug overdose, since knowledge of the change in her tricyclic plasma levels over time (Figure 1) was critical in determining what actually occurred in this case.
Often only a single tricyclic plasma level is obtained in such cases. Such a level is usually obtained either in the emergency room or shortly after admission to the hospital. This was the case with the first level obtained for this patient, which was 2040 ng/mL and, as discussed earlier, was fully consistent with an overdose. However, that level alone would not have distinguished an unintentional overdose due to a drug-drug interaction from an intentional overdose. Making this distinction required several additional plasma tricyclic levels obtained over a sufficient interval of time to determine the patient's clearance and compare that to what would have been expected in the usual patient. The markedly slower fall in the patient's levels (Figure 1) coupled with knowledge of the inhibitory effect of fluoxetine on amitriptyline supported the conclusion that this event was due to a drug-drug interaction rather than a suicide attempt.
The turnaround time for obtaining the levels in this case was more than a week. Although such a slow turnaround time is frustrating for the clinician, it was useful for the purposes of this column, because the initial diagnosis was based on the data that are usually available in such cases: a history of being treated for depression and physical and laboratory findings obtained in the emergency room or intensive care unit. Based on those data, the initial diagnosis in this case was a serious suicide attempt by acute overdose of amitriptyline. This conclusion was made despite the fact that the patient denied taking an overdose, her husband did not witness an overdose, and her pill bottle for amitriptyline was not missing pills. Two explanations were advanced for why no pills were missing: either the patient had stopped taking her amitriptyline some time before she took the overdose or she took the overdose from a previous supply of the medication since she had been on amitriptyline for some time. As a result of this conclusion, the patient was transferred to inpatient psychiatry.
This column thus adds to the literature documenting the need to consider drug-drug interactions in the differential diagnosis of sudden death due to intentional overdose. That literature includes the previous column on apparent acute overdose deaths resulting from the co-ingestion of fluvoxamine and other drugs.4 Another case has also been documented in which a fatal CYP-enzyme-mediated drug-drug interaction involving fluoxetine and amitriptyline was initially signed out as a suicide.15 In that case, as in this one, plasma drug levels (although obtained postmortem) were critical in determining that the death was actually the result of a prescriber-initiated unintentional overdose rather than a patient-initiated intentional overdose. This case thus provides another illustration of the difficulty of detecting drug-drug interactions even when they cause significant patient morbidity.
For completeness, one other point about this case should be noted. This patient was taking two combination products that both contained acetaminophen, but there was no evidence that the dose of these two medications had been adjusted to account for this fact. Acetaminophen produces dose-dependent liver toxicity. There is some evidence to suggest that such toxicity may be increased in individuals who consume alcohol, as this patient did. Although this is a more minor concern in this case, it illustrates a potential problem with such combination products and represents a third drug-drug interaction in this case: acetaminophen and alcohol.
The Hidden Cost of Drug-Drug Interactions
In addition to documenting the serious patient morbidity that can result from CYP-enzyme-mediated drug-drug interactions, this case illustrates the hidden cost associated with such interactions. In this case, two drug-drug interactions resulted in the patient's emergency transportation to the hospital, an evaluation in the emergency room, a stay of several days in an intensive care unit, and a subsequent stay of several days on a psychiatric unit. Fortunately, this patient fully recovered but she could have died. Nevertheless, the drug-drug interactions in this case caused a significant medical expenditure and would have gone undetected were it not for the data from repeated plasma drug levels (Figure 1) and a knowledge of the potential interactions involved in this case.
The hidden cost of drug-drug interactions has also been illustrated in several earlier columns16-18 and in other published case reports.19,20 These costs include emergency room evaluations, hospitalizations, nursing home placement, the addition of more medications, more outpatient visits, and more diagnostic tests. Many of these interventions, including ironically the addition of more medications, are made to treat the symptoms of a drug-drug interaction without recognizing that that is the cause of the problem.
The extent of the cost of unintended drug-drug interactions to the healthcare system is unknown. Case reports such as this one are useful in demonstrating that such interactions do occur and that they can present in a myriad of masked ways that sometimes make ascertainment quite difficult. Mathematical calculations of the number of potential drug combinations that can be used in clinical practice (2.8 x 1015) show the magnitude of the potential problem.21 Surveys of pharmacy records have documented that the extent and diversity of drug combinations used in clinical practice is substantial. For example, 76% of 5,000 patients selected solely on the basis of taking at least one systemically active prescription medication were on a unique regimen (i.e., no other patient in the sample was on the same regimen).22,23
All of the data presented here suggest that drug-drug interactions are a significant cost to the healthcare system as well as a significant cause of patient morbidity -- yet more work is needed to confirm this hypothesis and document the extent of the problem. However, a number of problems are inherent in doing such work. Such events are likely to present in many different ways, given the extent and uniqueness of polypharmacy used in clinical practice. This difficulty is further compounded by the fact that the adverse consequences may be recognized but their cause may not, so that the adverse event may not be coded in the medical record as being the result of a drug-drug interaction. All of these factors need to be considered when doing such research. Yet more data on the prevalence and types of drug-drug interactions that occur, coupled with more accurate and complete drug information, will help clinicians avoid untoward events such as the one illustrated in this case.