|
|
Outpatient Management of Depression
7 - Considerations When Selecting an Antidepressant |
|
|
|
|
|
As a basis for conceptualizing the pharmacology of the 22
different antidepressant options available in the United States,
Chapter 6 presented a classification
system which grouped all available antidepressants into eight
mechanistically defined classes. This chapter will translate
that basic pharmacology into five clinically relevant factors
to be considered when selecting any drug for any patient:
- Safety
- Tolerability
- Efficacy
- Payment
- Simplicity.
These five factors are summarized by the mnemonic, STEPS.
Each factor can be further subdivided as shown in Table
7.1. The discussion in this chapter will focus on each
factor and present a summary of how each antidepressant group
relates to that factor. A summary of the clinical pharmacology
of each class of antidepressants as they relate to these five
factors will be discussed in Chapter
8. A summary of how the various classes (and individual
members within these classes) compare on these five factors
is provided in Tables 7.2 through 7.9.
Safety
| TABLE 7.1
STEPS Criteria for Selecting an Antidepressant |
|
Safety
- Therapeutic index
- Drug-drug interactions
Pharmacodynamics
Pharmacokinetics
Tolerability
Efficacy
- Overall
- Unique spectrum of activity
- Rate of response
- Maintenance and prophylaxis
Payment
Simplicity
|
| Adapted from: Preskorn
SH. J Clin Psychiatry. 1994;55(suppl A):6-22,
23-24, 98-100. |
|
|
| TABLE 7.2 STEPS Criteria
for Mixed Reuptake and Neuroreceptor Antagonists
(eg, Amitriptyline) Safety |
|
Safety
Therapeutic index: narrow. Serious toxicity
can result from overdose, either acute ingestion
or from gradual accumulation due to slow clearance
Drug-drug interactions:
Pharmacodynamic: prone to cause multiple types
of such interactions due to their multiple mechanisms
of action (Table 6.3)
Pharmacokinetic: can be the target of such interactions
but are not prone to cause them. Due to narrow
therapeutic index, care should be taken when prescribing
TCAs to patients on drugs known to inhibit CYP
enzymes (Table 6.10)
Tolerability
Poor due to numerous types of adverse effects
mediated by the blockade of histamine receptors
(eg, sedation), muscarinic acetylcholine receptors
(eg, constipation), and alpha-1adrenergic receptors
(eg, orthostatic hypotension) (Tables 6.2, 6.3,
and 6.7)
Efficacy
Overall: highest response rates of any antidepressants.
Clomipramine in particular has been found to be
superior to two SSRIs, citalopram and paroxetine,
in hospitalized patients with clinical depression
Unique spectrum of activity: imipramine has
been found effective in patients who have not
benefited from treatment with the SSRI, sertraline
Rate of response: 2 to 4 weeks
Maintenance of response: established by controlled
studies Payment
Generic versions are available making these
antidepressants the least expensive in terms of
acquisition costs. These savings are likely offset
by expenses arising from problems with their safety
and tolerability (Table 7.10)
Simplicity
Must titrate dose due to tolerability problems.
Therapeutic drug monitoring should be done once
early in treatment to guide dose adjustment. Therapeutic
ranges have been established for most of these
TCAs. Can be given once a day
|
| Adapted from: Preskorn
SH. J Clin Psychiatry. 1994;55(suppl A):6-22,
23-24, 98-100. |
|
|
| TABLE 7.3 STEPS Criteria
for Norepinephrine Selective Reuptake Inhibitors
(eg, Desipramine) |
|
Safety
Therapeutic index: narrow. The only NSRIs available
at present in the United States are secondary
amine TCAs which have the same toxicity problems
as tertiary amine TCAs (eg, amitriptyline). However,
this safety problem is due to inhibition of fast
sodium channels as opposed to norepinephrine reuptake
inhibition. In fact, there are investigational,
nontricyclic NSRIs (eg, reboxetine) which do not
have a narrow therapeutic index in terms of cardiotoxicity
Drug-drug interactions:
Pharmacodynamic: limited at therapeutic concentrations
to those produced by norepinephrine potentiation
Pharmacokinetic: can be target of such interactions
but not prone to cause them. Due to narrow therapeutic
index, care should be taken when prescribing with
drugs known to inhibit CYP enzymes (Table 6.10)
Tolerability
Generally good. Adverse effects mediated by
norepinephrine potentiation (Tables 6.2, 6.3,
and 6.6)
Efficacy
Overall: comparable to tertiary amine TCAs
Unique spectrum of activity: have been found
in several studies to be effective in approximately
50% of patients who have not benefited from treatment
with a variety of SSRIs Rate of response: 2
to 4 weeks
Maintenance of response: established by controlled
studies Payment
Generic versions are available but cost almost
as much as newer antidepressants (Table 7.10)
Simplicity
Can start on an effective dose immediately.
Therapeutic drug monitoring should be done once
early in treatment to guide dose adjustment. Therapeutic
ranges have been established for most of these
TCAs. Can be given once a day
|
| Abbreviations: NSRI, norepinephrine
selective reuptake inhibitor; TCA, tricyclic antidepressant;
CYP, cytochrome P450 enzyme; SSRI, serotonin selective
reuptake inhibitor. |
|
|
| TABLE 7.4 STEPS Criteria
for Serotonin Selective Reuptake Inhibitors (eg,
Sertraline) |
|
Safety
Therapeutic index: wide. No serious systemic
toxicity demonstrated, even after substantial
overdose
Drug-drug interactions:
Pharmacodynamic: serotonin syndrome may occur
when used with other serotonin agonists. Can potentiate
dopamine antagonists in terms of extrapyramidal
effects (eg, motor restlessness)
Pharmacokinetic: considerable differences exist
among SSRIs in terms of their potential for decreasing
the rate of oxidative metabolism of a variety
of drugs by inhibiting CYP enzymes: fluoxetine,
fluvoxamine > paroxetine > citalopram, sertraline
(Table 6.10). No known clinically significant
effect of other drugs on the clearance of SSRIs
Tolerability
Good. Nausea and loose stools can occur early
and are dose dependent but tolerance typically
develops. Sexual dysfunction (eg, anorgasmia)
can occur in approximately 30% of patients (Tables
6.2, 6.3, and 6.6) Efficacy
Overall: equivalent to TCAs in outpatients
Unique spectrum of activity: can work in TCAs
nonresponders Rate of response: 2 to 4 weeks
Maintenance of response: evidence from controlled
studies Payment
Brand name only available; see discussion (Table
7.10)
Simplicity
Ease of administration: fluoxetine, paroxetine,
and sertraline can be started at an effective
dose immediately. Nonlinear pharmacokinetics of
paroxetine likely contribute to an increased risk
of experiencing an SRI discontinuation syndrome
after abrupt cessation. The long half-life of
fluoxetine and active metabolite, norfluoxetine,
means time to maximum effect and time to washout
can take up to 2 months
|
| Abbreviations: SSRI, serotonin
selective reuptake inhibitor; CYP, cytochrome P450
enzyme; TCA, tricyclic antidepressant; SRI, serotonin
reuptake inhibitor. |
|
|
| TABLE 7.5 STEPS Criteria
for Serotonin and Norepinephrine Reuptake Inhibitors
(eg, Venlafaxine) |
|
Safety
Therapeutic index: wide. No serious systemic
toxicity demonstrated. Dose-dependent hypertension
going from 1% above placebo at doses £ 100 mg/day
to 11% above placebo at doses ³ 300 mg/day. That
fact is consistent with NE uptake inhibition occurring
at higher doses
Drug-drug interaction:
Pharmacodynamic: same as SSRIs at low doses.
NE-mediated interactions possible at higher doses
Pharmacokinetic: has profile comparable to citalopram
and sertraline in terms of no to minimal effects
on most CYP enzymes (Table 6.10)
Tolerability
Comparable to SSRIs at low dose; NE-mediated
adverse effects at higher doses (Tables 6.2, 6.3,
and 6.7)
Efficacy
Overall: equivalent to TCAs. High-dose venlafaxine
was superior to fluoxetine in double-blind study
Unique spectrum of activity: possible efficacy
in cases not responsive to TCAs or SSRIs
Rate of response is a function of dose: 2 to
4 weeks at doses £ 150 mg/day and 4 to 7 days
at doses of ³ 300 mg/day
Maintenance of response: evidence from controlled
studies
Payment
Brand name only available; see discussion (Table
7.10)
Simplicity
Ease of administration: can be started at an
effective dosage (75 mg/day); once daily dosing
with sustained-release formulation. Dose titration
is not necessary for many patients but increasing
the dose is a reasonable strategy if starting
dose is ineffective
|
| Abbreviations: NE, norepinephrine;
SSRI, serotonin selective reuptake inhibitor; CYP,
cytochrome P450 enzyme; TCA, tricyclic antidepressant. |
|
|
| TABLE 7.6 STEPS Criteria
for Serotonin-2A Receptor Antagonist and Weak Serotonin
Reuptake Inhibitors (eg, Nefazodone) |
|
Safety
Therapeutic index: wide. No serious systemic
toxicity due to acute overdose
Drug-drug interactions:
Pharmacodynamic: can interact with other agents
that decrease arousal or impair cognitive performance.
Can interact with adrenergic agents affecting
blood pressure regulation. Complex interactions
with other serotonin-active agents
Pharmacokinetic: substantially inhibits CYP
3A which is responsible for 50% of known oxidative
drug metabolism, including its own metabolism
(ie, nonlinear pharmacokinetics or autoinhibition)
Tolerability
Dizziness, drowsiness, and confusion are dose-dependent
adverse effects; hence the need for dose titration
(Tables 6.2, 6.3, and 6.7)
Efficacy
Overall: equivalent to TCAs in outpatients
Unique spectrum of activity: none demonstrated
Rate of response: 2 to 4 weeks
Maintenance of response: evidence from controlled
studies Payment
Generic version of trazodone available. Brand
name only for nefazodone. The latter has clinically
important pharmacologic advantages over the former
(Table 7.10)
Simplicity
Ease of administration: requires dose titration
and divided daily dosing for optimal antidepressant
effect
Safety
Therapeutic index: wide. No serious systemic
toxicity due to acute overdose
Drug-drug interactions:
Pharmacodynamic: can interact with other agents
that decrease arousal or impair cognitive performance.
Can interact with adrenergic agents affecting
blood pressure regulation. Complex interactions
with other serotonin-active agents
Pharmacokinetic: substantially inhibits CYP
3A which is responsible for 50% of known oxidative
drug metabolism, including its own metabolism
(ie, nonlinear pharmacokinetics or autoinhibition)
Tolerability
Dizziness, drowsiness, and confusion are dose-dependent
adverse effects; hence the need for dose titration
(Tables 6.2, 6.3, and 6.7)
Efficacy
Overall: equivalent to TCAs in outpatients
Unique spectrum of activity: none demonstrated
Rate of response: 2 to 4 weeks
Maintenance of response: evidence from controlled
studies Payment
Generic version of trazodone available. Brand
name only for nefazodone. The latter has clinically
important pharmacologic advantages over the former
(Table 7.10)
Simplicity
Ease of administration: requires dose titration
and divided daily dosing for optimal antidepressant
effect
|
| Abbreviations:
CYP, cytochrome P450 enzyme; TCA, tricyclic antidepressant. |
|
|
| TABLE 7.7 STEPS Criteria
for Serotonin (5-HT2A and 5-HT2C) and Norepinephrine
(a-2) Receptor Antagonists (eg, Mirtazapine) |
|
Safety
Therapeutic index: wide in terms of acute drug
overdose. There were three cases of agranulocytosis
out of approximately 3000 patients in clinical
trial program. That number was too small to establish
with confidence the actual incidence or even whether
there was a causal relation. Postmarketing experience
has not indicated that toxicity is a major concern.
Nevertheless, a white count should be obtained
if a patient presents with signs of fever or infection
Drug-drug interaction:
Pharmacodynamic: can cause multiple types of
such interactions due to multiple mechanisms of
action (Table 6.3)
Pharmacokinetic: unlikely to either be a victim
or a cause of such interactions based on in vitro
studies, but that should be confirmed by in vivo
studies
Tolerability
Primary problems are sedation due to histamine
receptor blockade and weight gain due to 5-HT2C
receptor blockade. These can be treatment limiting
(Tables 6.2, 6.3, and 6.7). Dose titration may
not help
Efficacy
Overall: good; was superior to fluoxetine in
double-blind study of hospitalized patients
Unique spectrum of activity: worked in 54% of
patients who had not benefited from treatment
with amitriptyline. Open label studies have reported
effectiveness in patients who had not benefited
from SSRIs
Rate of response: 2 to 4 weeks
Maintenance of response: data limited to noncontrolled
open label extension studies
Payment
Brand name only available; see discussion (Table
7.10)
Simplicity
Can start on an effective dose immediately.
Sedation can be a problem, but starting with a
lower dose may only decrease efficacy without
improving tolerability, because mirtazapine is
a more potent histamine receptor blocker than
it is a serotonin and adrenergic receptor blocker.
No rigorous data on whether higher doses increase
either efficacy or tolerability
|
|
|
| TABLE 7.8 STEPS Criteria
for Dopamine and Norepinephrine Reuptake Inhibitors
(eg, Bupropion) |
|
Safety
Therapeutic index: narrow. The dose needed for
efficacy (300-450 mg/day) is only 2 to 3 times
less than the dose that causes seizures in 2%
of patients. That is the reason the maximum recommended
daily dose is 450 mg/day. Seizures can also occur
following an acute overdose but are generally
managed easily in a medical setting
Drug-drug interaction:
Pharmacodynamic: should potentiate and reduce
the effects of other dopamine and norepinephrine
agonists and antagonists, respectively
Pharmacokinetic: can be affected by fluoxetine
and probably others in a clinically significant
way. Due to its narrow therapeutic index, care
should be taken when prescribing bupropion to
patients on drugs known to inhibit CYP enzymes.
Case report data suggest that it can substantially
inhibit CYP 2D6
Tolerability
Good. Does not cause sexual dysfunction seen
with antidepressants which are serotonin reuptake
inhibitors (Tables 6.2, 6.3, and 6.7)
Efficacy
Overall: probably less than TCAs Unique spectrum
of activity: can work in TCA nonresponders
Rate of response: 2 to 4 weeks
Maintenance of response: not demonstrated in
formal studies
Payment
Brand name only available; see discussion (Table
7.10)
Simplicity
Ease of administration: requires divided daily
dosing for antidepressant effect and dose titration
to achieve efficacy and minimize seizure risk
|
| Abbreviations: CYP, cytochrome
P450 enzyme; TCA, tricyclic antidepressant. |
|
|
| TABLE 7.9
STEPS Criteria for Monoamine Oxidase Inhibitors
(eg, Tranylcypromine) |
|
Safety
Therapeutic index: narrow. Serious toxicity
can result from acute overdose
Drug-drug interaction:
Pharmacodynamic: hypertensive crisis can result
from coadministration with tyramine and sympathomimetic
agents. Serotonin syndrome can result from coadministration
with serotonin agonists (eg, serotonin uptake
inhibitors) (Table 6.2)
Pharmacokinetic: inhibits the oxidative enzyme,
MAOI, but effects on CYP enzymes have not been
studied. Not known to be affected by other drugs
in a clinically significant way
Tolerability
Generally good, especially if kept to effective
minimum dose. Ironically, main tolerability problem
is hypotension (Table 6.3)
Efficacy
Overall: Probably less than TCAs
Unique spectrum of activity: can work in TCA
nonresponders
Rate of response: 2 to 4 weeks
Maintenance of response: not adequately tested
Payment
Generic versions are available making these
antidepressants the least expensive in terms of
acquisition costs (Table 7.10). These savings
are likely offset by expenses arising from problems
with their safety and tolerability
Simplicity
Ease of administration: typically administered
in divided daily doses. Dose titration recommended
to optimize efficacy and minimize hypotension
|
| Abbreviations: MAOI, monoamine
oxidase inhibitor; CYP, cytochrome P450 enzyme;
TCA, tricyclic antidepressant. |
|
Tertiaryamine tricyclic antidepressants
(TATCAs) have a narrow acute therapeutic index due to their
inhibition of sodium (Na+) fast channels at concentrations
only 10 times higher than those needed to treat major depression.194
For this reason, an overdose of these drugs carries a serious
risk of slowing intracardiac conduction to the point of inducing
a fatal ventricular arrhythmia. This effect also occurs with
secondary amine TCAs (eg, desipramine) even though these antidepressants
are norepinephrine selective reuptake inhibitors (NSRIs) at
usual therapeutic concentrations (Table
6.2). Other than TCAs, fatal overdose is not an issue
with any other antidepressant when taken alone since they
do not affect intracardiac conduction.
Some clinicians mistakenly believe that
the arrhythmia caused by antidepressants such as desipramine
is due to their ability to inhibit the neuronal uptake pump
for norepinephrine. However, reboxetine (an investigational
non-TCA NSRI) and venlafaxine (a serotonin and norepinephrine
reuptake inhibitor [SNRI]) block norepinephrine uptake, but
do not inhibit Na+ fast channels even at concentrations achieved
following a moderate overdose. Arrhythmias generally do not
occur following overdose of these norepinephrine-active antidepressants.41,73
Although not as serious as the cardiotoxicity of TCAs, there
are some safety concerns with some newer antidepressants.
For example, bupropion has a dose-dependent risk of seizures.57
At dosages of 450 mg/day, the seizure risk is 0.4%; that risk
may be lower with the sustained-release formulations because
of the blunting of peak levels. The risk of seizures due to
bupropion increases substantially when its dosage exceeds
450 mg/day (the maximum recommended daily dosage).
Consistent with their pharmacology as indirect sympathomimetic
agonists, NSRIs (eg, desipramine) and high-dosages of the
SNRI venlafaxine (ie, > 225 mg/day) can cause dose-dependent
increases in blood pressure.73,169,240
The magnitude of the increases in blood pressure produced
by these drugs is generally modest, but in some cases, can
be sufficient to warrant either discontinuation or the addition
of an antihypertensive medication.
Finally, some newer antidepressants (eg, fluoxetine) at
their usually effective antidepressant dose produce substantial
inhibition of one or more drug metabolizing cytochrome P450
(CYP) enzymes (Table 6.10
and Figure 6.2). That action
carries with it the liability for causing clinically significant
and even fatal pharmacokinetically mediated drug-drug interactions
(Chapter 10).
Antidepressants are among the most widely
prescribed medications and are often taken on a long-term
basis to prevent the recurrence of major depressive episodes.
There is no evidence of serious long-term safety problems
with any antidepressant, based on spontaneous reports to the
Food and Drug Administration (FDA). The long-term safety of
antidepressants has also been systemically evaluated during
clinical trial development programs.103,169,178
The most rigorous data in this regard come from year-long,
placebo-controlled relapse prevention studies.62,67,112,148
Other data come from open-label extension studies. In these
studies, patients who have completed double-blind acute treatment
studies can elect to continue on the medication until it is
approved by the FDA. Given these two types of approaches,
the safety of the drug will have typically been assessed in
several hundred patients for up to 1 year by the time it is
available in the United States.
- Pharmacodynamic Interactions
The more sites of action affected by a drug, the more potential
there is for pharmacodynamic interaction with other coprescribed
drugs.192 In fact,
the sites of action affected by a drug determine the specific
type of pharmacodynamic drug-drug interaction(s) it can cause.
Tertiary amine TCAs, due to their multiple effects on different
neural targets (Table 6.2),
will cause the most types of pharmacodynamic drug-drug interactions.192
Specifically, these antidepressants can:
- Potentiate the sedative effects of alcohol and other sedative
hypnotics via their blockade of the histamine-1 receptor
- Enhance the antiperistaltic effects of other drugs via
their blockade of the muscarinic cholinergic receptor
- Increase the blood pressure-lowering effects of a variety
of antihypertensives via their blockade of the alpha-1-adrenergic
receptor
- Potentiate the slowing of intracardiac
conduction produced by various antiarrhythmic agents via
their inhibition of Na+ fast channels
- Cause a hypertensive crisis when used with other norepinephrine
agonists like monoamine oxidase inhibitors (MAOIs) due to
their ability to inhibit the norepinephrine uptake pump
- Cause a serotonin syndrome when used with other serotonin
agonists like MAOIs due to their ability to inhibit the
serotonin uptake pump.
Using Tables 6.2 and 6.3,
the clinician can determine which other antidepressants share
the same potential to cause specific types of pharmacodynamic
drug interactions as do the TATCAs.
- Pharmacokinetic Interactions
The CYP enzyme-mediated drug-drug interactions are the most
common type of pharmacokinetic drug-drug interaction.93,94
The clinician can use Table
6.10 to determine which antidepressants are most likely
to cause such interactions (ie, be the perpetrator). Table
6.9 can be used to determine which drugs will be affected
(ie, be the victim) by an alteration (ie, induction or inhibition)
in the metabolic capacity of a specific CYP enzyme. Fluoxetine
and fluvoxamine will cause the most types of CYP enzyme-mediated
drug interactions because they inhibit more than one CYP enzyme
to a substantial degree at their usually effective antidepressant
dose (Table 6.10 and Figure
6.2).
Pharmacokinetic drug interactions can
present in many different ways (ie, decreased tolerability,
decreased efficacy, withdrawal syndromes, or increased toxicity)
because they typically produce an effect comparable to a change
in the dose of the affected drug (Chapter
10). The clinician may not realize that starting or stopping
the inhibitor has effectively changed the functional dose
of the affected or victim drug by changing its clearance.
Hence, the change in efficacy or tolerability may incorrectly
be attributed to a patient sensitivity problem or to an underlying
medical problem. Such misdiagnosis can:
- Prolong the patient's suffering
- Increase the cost of health care
- Complicate prescriber's management of patient.
Tolerability
- Acute Tolerability Problems
As with drug-drug interactions, the more sites of action
that a drug affects, the more types of adverse effects it
can produce. The clinician can use Tables 6.2
and 6.3 to predict the types
of adverse effects of each class of antidepressant. Tables
6.4 through 6.7
contain frequency of specific adverse effects for various
antidepressants based on double-blind, placebo-controlled
studies. Chapter 11 discusses the
possible management approaches to the most common nuisance
adverse effects of specific antidepressants.
- Long-Term Tolerability Problems
Although most of the adverse effects caused by antidepressants
occur after the first dose, or at least within the first week
of treatment, sexual dysfunction is an exception.145,147
This effect frequently does not come to clinical attention
until several weeks or months into treatment. All antidepressants
that produce substantial serotonin uptake inhibition can cause:
- Anorgasmia
- Decreased libido
- Delayed ejaculation.145,147
There is no universally effective treatment
for the sexual dysfunction caused by serotonin uptake inhibitors,
but potential useful "antidotes" are discussed in
Chapter 11.
Other types of sexual dysfunction caused by antidepressants
include:
- Tertiary amine TCAs can cause impotence via their alpha-1-adrenergic
blockade.103
- Trazodone can cause priapism in approximately 1 in 8000
males.103
Norepinephrine uptake inhibitors (eg, desipramine), bupropion,
mirtazapine and nefazodone at dosages below 500 mg/day appear
to have minimal risk of causing sexual adverse effects.103,151,171,213
Efficacy
As with many other illnesses, the treatment of clinical depression
can be divided into several clinically important and distinct
phases as follows:
- Acute induction of remission
- Maintenance of remission during the vulnerable period
of time for a relapse
- Prophylactic treatment to protect against future depressive
episodes.4,61,98
There are several factors that need to be weighed when considering
the induction of an acute remission as follows:
- Overall efficacy
- Unique spectrum of efficacy
- Rate of response.169
Clinical depression is a syndrome that
will likely prove to be more than one illness when understood
from the perspective of pathophysiology and/or etiology. The
results of genetic and familial studies, biological marker
studies, psychosocial studies and antidepressant clinical
trials are all consistent with the conclusion that clinical
depression is a heterogeneous group of disorders. That means
that antidepressants with different mechanisms of action may
treat different types of clinical depression (ie, have different
overall acute efficacy and different spectra of efficacy).
Overall acute efficacy refers to how many patients
with clinical depression will respond to a trial of a particular
antidepressant. Assuming all other factors are equal (ie,
safety, tolerability, cost and simplicity), the antidepressant
of first choice would be the one that will produce a clinically
meaningful, acute response in the largest number of patients.
Unique spectrum of efficacy refers to whether an
antidepressant will work in a patient who has not benefited
from a previous trial of another antidepressant. This issue
is clinically important because virtually any antidepressant
fails to produce an adequate response in one third to one
half of patients suffering from clinical depression.
Rate of response is self-explanatory. This issue is
more of interest to psychiatrists than to the primary-care
practitioner, since the usual time course for response to
an antidepressant (ie, approximately 2 weeks) does not typically
pose a significant problem for outpatients with mild-to-moderate
clinical depression.
Maintenance efficacy refers to whether there is evidence
that continued treatment with the antidepressant is an effective
strategy to prevent a relapse of the current episode. Since
clinical depression is a recurrent condition in 50% or more
of cases, prophylactic efficacy refers to whether there
is evidence that indefinite treatment with an antidepressant
in high-risk patients can prevent future episodes.
There is some evidence that drugs having more than one mechanism
of antidepressant action may produce a higher percentage of
response than do antidepressants with a single mechanism of
action. Specifically, there are double-blind studies in which
clomipramine (a TATCA), mirtazapine and venlafaxine produced
a statistically significant better response rate than did
an active control with a single mechanism of action (eg, serotonin
uptake inhibition) in hospitalized patients with severe clinical
depression.48,55,56,267
On the other hand, antidepressants with multiple mechanisms
of action often cause a higher dropout rate due to an increased
incidence of adverse effects. Thus, their increased efficacy
can be offset by the increased dropout rate such that the
net outcome is a "wash."
Most antidepressants take at least 2
weeks to demonstrate a higher response rate than the parallel
placebo-controlled condition.103
In fact, it is advisable to not give up on an antidepressant
or even to increase the dose of the single-mechanism-of-action
antidepressant until a trial of at least 4 weeks on a stable
dose has been given. In contrast, there is one study indicating
that high-dosage venlafaxine (ie, > 300 mg/day) can produce
a response in 20% of patients within 1 week and can statistically
separate from placebo as soon as 4 days.169
These findings are consistent with combined inhibition of
both serotonin and norepinephrine uptake pumps at such doses
of venlafaxine (Figure 6.4).103
A similar rapid onset of antidepressant action has also been
produced by using a combination of an NSRI (eg, desipramine)
and a serotonin selection reuptake inhibitor (SSRI) (eg, sertraline).153,258
Of course, either of these approaches (ie, high dose venlafaxine
or combined use of an NSRI and an SSRI) to increase efficacy
by affecting these two mechanisms of action sets the stage
for more types of adverse effects mediated by these two different
mechanisms of action (Table 6.5).
The increase in tolerability problems can more than offset
the increase in efficacy, particularly in the primary-care
setting where there is typically no need for a more rapid
response than is achieved with conventional antidepressant
treatment. Nevertheless, these strategies can be useful when
dealing with a severely ill or hospitalized patient where
time of response is a critical issue.
- Unique Spectrum of Efficacy
One advantage of having eight mechanistically different
classes of antidepressants is the possibility that patients
who are intolerant of or do not respond to one type of antidepressant
may benefit from treatment with a mechanistically different
type of antidepressant. While this possibility is intuitively
plausible, this subject has received scant systematic study
(Chapter 11). Most studies that
have tested this possibility have not been scientifically
rigorous, but were instead open label and hence subject to
potential biases.
The best data on this topic come from a series of double-blind,
crossover studies with NSRIs and SSRIs.245
Based on these studies, approximately 50% of patients who
do not benefit from a trial of an SSRI will benefit when switched
to an NSRI. The reverse is also true. This evidence is also
fully consistent with the aforementioned data that combined
treatment with an NSRI and an SSRI or with high-dose venlafaxine
produces more responders than does treatment with either mechanism
alone. While the available data are modest, there certainly
is no evidence to suggest that a nonresponse to one type of
antidepressant predicts nonresponse to a mechanistically different
type of antidepressant.61,245
- Maintenance and
Prophylactic Efficacy
The FDA does not require proof of either maintenance or
prophylactic efficacy to approve a drug as an antidepressant.
Nevertheless, most approved newer antidepressants have been
tested and shown to have sustained efficacy over the maintenance
phase (ie, 6 months after the induction of an acute remission).
The best studies involve open-label treatment for 2 to 3 months
to induce an acute response and then double-blind, random
assignment to either remain on the antidepressant or be switched
to placebo.62,67,148
Approximately one fourth to one third more patients on placebo
will relapse back into their depressive episode over a 6-month
interval in comparison to those maintained on the antidepressant.
These data are sufficiently compelling so that continued treatment
with an antidepressant for 6 months after an acute response
is recommended for all first-episode patients.4,61
Less well studied is the issue of prophylactic efficacy.
The single best study has been done with the TATCA, imipramine.76
The follow-up treatment period for most patients in this study
was 3 years, but some were followed for 5 years. The patients
were selected for being at high risk for relapse. Participants
were required to have had at least four episodes of depression
(ie, the current one plus three prior episodes). The results
provided compelling support for the benefit of prophylactic
treatment with an antidepressant in such a high risk population-only
20% of the patients who remained on imipramine relapsed at
the end of 3 years versus 95% of patients who were switched
to placebo. A more recent 76-week, double-blind, randomized,
parallel-group study demonstrated the superiority of sertraline
over placebo in the maintenance treatment of patients with
chronic major depression.112
Studies with other antidepressants have
generally not lasted more than 1 year and have not been conducted
in patients with such a high risk for recurrent episodes.
Nevertheless, these studies have also demonstrated fewer recurrent
episodes on drug versus a parallel, placebo-controlled condition.
Payment
This issue is complex, controversial, and not unique to antidepressants.
Clinicians often find themselves between two competing forces:
- The pharmaceutical company, which develops a new medication
- The patient or their third-party insurer, who pays for
the medication.
The latter is generally concerned with the acquisition cost
(ie, how much does the prescription cost) (Table
7.10). The former points out that the increased acquisition
cost of the new medication is more than offset by other "hidden"
costs of the older and often generic drugs.
For example, the acquisition cost of a TATCA is considerably
less than all of the newer antidepressants, but there are
other associated costs that should be taken into account when
doing a cost comparison.40
These include but are not limited to:
- More physician visits
- Additional laboratory tests. For example, therapeutic
drug monitoring should be done at least once when prescribing
TCAs due to their narrow therapeutic index and high interindividual
variability in clearance
- In the case of TCAs, there is also the cost of intensive
care for the patient who takes an acute overdose
- Cost of treating adverse effects
- Tolerability problems may also have
a hidden cost if the patient relapses because the medication
was stopped too soon due to a persistent adverse effect.
Simplicity
An important issue when selecting an antidepressant is how
easily can the clinician select an optimal dose for the patient;
that is, determine the dose which has the greatest likelihood
of producing a good antidepressant response and the least
risk of causing either nuisance or serious adverse effects
(Table 7.11).169
This issue is particularly important for the primary-care
practitioner given time constraints in daily practice.
A related issue is how easy is it for the patient to take
the medication as prescribed. An ideal drug would be one that:
- Can be started at an effective dose (ie, no need for titration)
- Optimum dose easily determined
- Requires no special laboratory testing to guard against
toxicity either before or during treatment
- Can be taken once daily.
Start At Effective Dose
Several of the antidepressants meet many
of the requirements above. Most SSRIs (ie, fluoxetine, paroxetine,
and sertraline, but not citalopram and fluvoxamine), mirtazapine,
most NSRIs (eg, desipramine, nortriptyline), and venlafaxine
can be started at an effective dose. A few additional comments
are warranted with regard to mirtazapine. Sedation can be
a problem in early treatment with mirtazapine.178
For this reason, some clinicians may consider starting with
a lower dose (7.5 mg), but this strategy may not avoid sedation
(the drug's most common effect [Table
6.7]) and may compromise efficacy.151
In contrast to the above antidepressants, bupropion, nefazodone,
and TATCAs do require dose titration primarily to minimize
adverse effects.103
In the case of bupropion, the goal of titration is to find
the lowest effective dose to minimize the risk of seizures.57
In the case of nefazodone and TATCAs, the issue is minimizing
nonserious but nevertheless nuisance and sometimes rate-limiting
adverse effects.
|
| TABLE 7.10
Average Wholesale Price of Representative Doses
of Antidepressants* |
| Generic/Trade
Drug Name |
Strength
(mg) |
Price
($/100) |
| Generic
|
Trade |
| Tertiary
Amine Tricyclic Antidepressants |
|
| Amitriptyline/Elavill |
25 |
10 |
40 |
| 50 |
12 |
72 |
| 100 |
18 |
125 |
| Doxepin/Sinequan |
25 |
14 |
47 |
| 50 |
19 |
65 |
| 100 |
40 |
119 |
| Imipramine/Tofranil |
25 |
6 |
47 |
| 50 |
9 |
80 |
| Selective
Norepinephrine Reuptake Inhibitors |
|
| Desipramine/Norpramin |
25 |
25 |
65 |
| 50 |
50 |
122 |
| 100 |
100 |
204 |
| Nortriptyline/Pamelor |
25 |
80 |
100 |
| 50 |
150 |
190 |
| Serotonin
Selective Reuptake Inhibitors |
|
| Citalopram/Celexa |
20 |
NA |
193 |
| 40 |
NA |
202 |
| Fluoxetine/Prozac |
10 |
NA |
230 |
| 20 |
NA |
240 |
| Fluvoxamine/Luvox |
50 |
NA |
206 |
| 100 |
NA |
212 |
| Paroxetine/Paxil |
10 |
NA |
190 |
| 20 |
NA |
200 |
| 30 |
NA |
210 |
| 40 |
NA |
222 |
| Sertraline/Zoloft |
50 |
NA |
180 |
| 100 |
NA |
200 |
| Serotonin
and Norepineprhine Reuptake Inhibitors |
|
| Venlafaxine-IR/Effexor
IR |
37.5 |
NA |
105 |
| 75 |
NA |
115 |
| 100 |
NA |
122 |
| Venlafaxine-XR/Effexor
XR |
37.5 |
NA |
194 |
| 75 |
NA |
217 |
| 150 |
NA |
237 |
| Serotonin
2A Antagonists |
|
| Nefazodone/Serzone |
100,
150, 200, 250 |
NA |
93 |
| Trazodone/Desyrel |
50 |
30 |
150 |
| 100 |
40 |
250 |
| 300 |
NA |
390 |
Serotonin
(5-HT2A and 2C) and Adrenergic (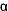 -2)
Antagonists -2)
Antagonists |
|
| Mirtazapine/Remeron |
15 |
NA |
195 |
| 30 |
NA |
202 |
| Dopamine
and Norepinephrine Reuptake Inhibitors |
|
| Bupropion-IR/Wellbutrin
IR |
75 |
NA |
60 |
| 100 |
NA |
83 |
| Bupropion-SR/Wellbutrin
SR |
100 |
NA |
115 |
| 150 |
NA |
117 |
| Monoamine
Oxidase Inhibitors |
|
| Phenelzine/Nardil |
15 |
NA |
42 |
| Tranylcypromine/Parnate |
10 |
NA |
50 |
| Abbreviations:
NA, none available; IR, immediate release; SR, sustained
release; XR, extended release. |
| * Information
taken from 1998 Drug Topics Red Book and
rounded to the nearest dollar. Recommended dosing
is twice or three times a day, whereas other drugs
in this chart are given once a day. That difference
will affect the daily cost of treatment (ie, how
long 100 pills will last). |
|
|
| TABLE 7.11
Summary of Package Insert Dosing Guidelines |
| Generic/Trade
Drug Name |
Recommended
Dose
Start/ Max (mg/day) |
Dosage Guidelines
for Specific Patients |
| Children |
Adolescents |
Elderly |
Hepatic* |
Renal* |
| Mixed Reuptake
Inhibitors and Neuroreceptor Blockersa,b |
|
| Amitriptyline/Elavil |
75/300c |
 |
NA |
 |
 |
 |
| Amoxapine/Ascendin |
100/600c,d |
NR |
NA |
 |
 |
 |
| Clomipramine/Anafranile |
25/250c,f |
NA |
NA |
 |
 |
 |
| Doxepin/Sinequan
|
75/300c |
NA |
NA |
NA |
 |
 |
| Imipramine/Tofranil |
75/300cc |
NA |
 |
 |
 |
 |
| Norepinephrine
Selective Reuptake Inhibitorsa,b |
|
| Desipramine/Norpramin |
100/300c |
 |
NA |
 |
 |
 |
| Maprotiline/Ludiomil |
75/225c,f |
NA |
NA |
 |
 |
 |
| Nortriptyline/Pamelor |
50/150c |
NA |
NA |
 |
 |
 |
| Serotonin
Selective Reuptake Inhibitors |
|
| Citalopram/Celexa |
20/60 |
NA |
NA |
 |
 |
 |
| Fluoxetine/Prozac |
20/80 |
NA |
NA |
 |
 |
 |
| Fluvoxamine/Luvoxe |
50/300g |
NR |
NA |
 |
 |
 |
| Paroxetine/Paxil |
20/50 |
NA |
NA |
 |
 |
 |
| Sertraline/Zoloft |
50/200 |
 |
Same |
Same |
 |
 |
| Serotonin
and Norepinephrine Reuptake Inhibitors |
|
| Venlafaxine-IR/Effexor
IR |
75g,h/375g |
NA |
NA |
NA |
 |
 |
| Venlafaxine-XR/Effexor
XR |
75h/375 |
NA |
NA |
NA |
 |
 |
| Serotonin
(5-HT2A) Receptor Blockers and Weak Serotonin Uptake
Inhibitors |
|
| Nefazodone/Serzone |
200g/600g |
NA |
NA |
 |
 |
 |
| Trazodone/Dyserel |
150g/600g |
NA |
NA |
 |
 |
 |
| Serotonin
(5-HT2A and 5-HT2C) and Norepinephrine Receptor
Blockers |
|
| Mirtazapine/Remeron |
15/45 |
NA |
NA |
 |
 |
 |
| Dopamine
and Norepinephrine Reuptake Inhibitors |
|
| Bupropion-IR/Wellbutrin
IR |
200g/450c,f,i |
NA |
NA |
 |
 |
 |
| Bupropion-SR/Wellbutrin
SR |
150/400c,f,i |
NA |
NA |
 |
 |
 |
| Monoamine
Oxidase Inhibitors |
|
| Phenelzine/Nardil |
45g/90g |
NA |
NA |
 |
 |
 |
| Tranylcypromine/Parnate |
30g/60g |
NA |
NA |
 |
 |
 |
| Abbreviations:
NA, not available; NR, not recommended; IR, immediate
release; XR, extended release; SR, sustained release. |
| * Impairment. |
- Starting dose may be given either as a once-a-day
dose or on a divided schedule. Once an effective
and tolerated dose has been established, it
may be given on a once-a-day basis, but a divided
dose may still be more prudent with a higher
total dose and in patients who are elderly or
debilitated. The maximum once-a-day dose of
doxepin is 150 mg.
- Usual dose may be given either as a once-a-day
dose or on a divided schedule.
- Therapeutic drug monitoring has been either
demonstrated to increase the safe and efficacious
use of this drug or theoretically should; demonstrated
for amitriptyline, clomipramine, desipramine,
imipramine, and nortriptyline. Theoretical for
the rest, but has not been adequately studied.
- Doses should exceed 400 mg/day only in hospitalized
patients who do not have a history of seizures
and who have not benefited from an adequate
trial of 400 mg/day.
- Not formally labeled by the FDA for the treatment
of clinical depression but rather for obsessive-compulsive
disorder; labeled for use as an antidepressant
in other countries.
- Maximum daily dose should not be exceeded
due to an increased risk of seizures.
- Dose should be given on a divided schedule
(bid or tid).
- For some patients, it may be desirable to
start at half the dose for 4 to 7 days to improve
tolerance, particularly in terms of nausea.
- It is particularly important to administer
in a manner most likely to minimize the risk
of seizures. Dose increases should not exceed
100 mg/day in a 3-day period. Cautious dose
titration can also minimize agitation, motor
restlessness, and insomnia. Time between doses
should be at least 4 hours for 100 mg IR doses,
6 hours for 150 mg IR doses, and 8 hours for
SR doses. Increases above 300 mg/day should
only be done in patients with no clinical effects
after several weeks of treatment at 300 mg/day.
Bupropion should be discontinued in patients
who do not experience an adequate response after
an adequate period on maximum recommended daily
dose. Dosing in the elderly, the debilitated,
and patients with hepatic and/or renal impairment
has not been adequately studied so increased
caution may be prudent.
|
|
Additional comments on dose titration: The package
inserts for the following drugs indicate that
they can be started at a dose which is usually
effective to treat clinical depression: fluoxetine,
mirtazapine, paroxetine, tranylcypromine, sertraline,
venlafaxine. The following comments apply about
the use of higher doses with these antidepressants.
For fluoxetine, paroxetine, and sertraline:
although fixed-dose studies in patients with clinical
depression found no advantage on average to higher
doses, an increase may be considered after several
weeks on the starting dose if no clinical improvement
has been observed. For mirtazapine, dose escalation
should not be made at intervals of less than 1
to 2 weeks to adequately evaluate therapeutic
response to a given dose. For tranylcypromine,
improvement can be seen between 48 hours and 2
weeks of starting therapy; if not, dose increases
in 10 mg/day increments may be made at intervals
of 1 to 3 weeks.
The package inserts for the following drugs recommend
starting at a lower than usually effective dose
and titrate up to a dose which is usually effective
to treat clinical depression in order to minimize
tolerability or safety problems: amitriptyline,
amoxapine, bupropion, citalopram, clomipramine,
fluvoxamine, doxepin, imipramine, nefazodone,
phenelzine, trazodone, and trimipramine. The following
are additional comments about dose titration with
these antidepressants: For the tricyclic antidepressants,
the dose should be gradually increased during
the first 2 weeks based on therapeutic drug monitoring
and clinical assessment of efficacy and tolerability.
For fluvoxamine, a lower than usually effective
starting dose is recommended to improve tolerability.
The dose should be increased every 4 to 7 days
as tolerated until maximum therapeutic benefit
is achieved. For citalopram, the starting
dose is 20 mg/day with the recommendation to generally
increase to 40 mg/day. While doses above 40 mg/day
are not ordinarily recommended, some patients
may require a dose of 60 mg/day. For nefazodone,
a lower than usually effective starting dose is
recommended to improve tolerability. Dose titration
should occur in increments of 100 to 200 mg/day
as determined by tolerability and the need for
further clinical improvement. These incremental
advances should be done using divided doses and
at intervals of at least 1 week. It may be advisable
to titrate up more slowly in elderly and debilitated
patients. For phenelzine, a lower than
usually effective starting dose is recommended
to improve tolerability. Its dose should be increased
to at least 60 mg/day at a fairly rapid pace consistent
with good tolerability. For trazodone,
the same comments apply as for nefazodone when
trazodone is used as an antidepressant; however,
it is now mainly used as a nonhabit-forming sedative
given as a single bedtime dose of 50 to 200 mg
as needed for sleep.
|
|
The next issue is how easily the optimal dose of the antidepressant
can be determined. Therapeutic plasma level ranges have been
established for most TCAs. Thus, the clinician can use therapeutic
drug monitoring (TDM) to adjust the dosage to compensate for
interindividual differences in clearance in order to optimize
the likelihood of antidepressant efficacy while simultaneously
avoiding toxicity.191
While increasing efficacy is an advantage of TDM with TCAs,
the principal and compelling reason to use TDM with TCAs is
to avoid toxicity in slow metabolizers. This is not an issue
with any other class of antidepressants with the possible
exception of bupropion.180
In the case of the SSRI class, there is no advantage to
using higher than the usually effective minimum dose.170
This finding is based on the results of double-blind, fixed-dose
studies which have found flat dose-response curves above the
usually effective minimum dose for these antidepressants.170
This finding does not preclude the possibility that some patients
may benefit from a higher dose of an SSRI (Chapter
11). Although beyond the scope of this book, paroxetine
is the only SSRI for which there is substantial evidence that
higher doses are needed to treat patients with anxiety disorders
versus patients with clinical depression. The reasons for
this dosing difference are not clear.
In contrast to SSRIs, there is an ascending
dose-antidepressant response curve with venlafaxine.177
This antidepressant at its starting dosage of 75 mg/day produces
approximately the same number of responders as do the SSRIs,
but the percentage of responders increase with higher doses
consistent with its apparent dual mechanism of antidepressant
action (ie, serotonin and then norepinephrine uptake inhibition).
Consistent with its pharmacology, higher doses of venlafaxine
also cause a higher incidence of serotonin- and norepinephrine-mediated
adverse effects, including the potential to increase blood
pressure as previously discussed.
There are no published fixed-dose studies which empirically
establish that doses of mirtazapine higher than 15 mg/day
are more effective or better tolerated. There are anecdotal
and theoretical reasons to suspect that higher doses will
decrease the problem of sedation (ie, offsetting arousal effects
as a result of alpha-2-adrenergic blockade at higher concentrations
[Figure 6.4]). However, in
the case of bupropion and nefazodone, the optimal dose in
terms of efficacy and tolerability is quite variable and requires
empiric dose titration.103
Most of the antidepressants can be given once a day. The
exceptions are bupropion (including the sustained-release
formulation) and nefazodone which require at least twice-a-day
dosing (Table 7.11). While once-a-day
dosing is desirable, the available literature suggests that
problems with compliance do not become an issue until the
required dosing frequency is 3 times a day or more.
|

