|
|
Clinical Pharmacology of SSRI's
4 - How SSRIs as a Group Differ From TCAs |
|
|
|
|
|
The goal behind the rational development of selective serotonin
reuptake inhibitors (SSRIs) is to:
- Either maintain or ideally enhance antidepressant efficacy
- Increase the therapeutic index (ie, the safety margin
between the effective versus the toxic dose)
- Improve the tolerability profile
- Reduce the risk of pharmacodynamically mediated drug-drug
interactions
This development plan has succeeded relative to tricyclic
antidepressants (TCAs). Hence, SSRIs, for many physicians,
have replaced TCAs as antidepressants of first choice in the
treatment of patients with major depression. They also have
increased the likelihood that a patient with symptoms of major
depression will receive a trial of an antidepressant at an
optimal dose. The reason for the increased likelihood of a
trial of antidepressant is that physicians are more comfortable
giving an empirical trial of SSRIs than a trial of TCAs due
to their wider safety margin and better tolerability. The
likelihood that an optimal dose will be used is that there
is no need to titrate the dose of SSRIs for most patients.
The starting dose is the optimal for most patients. Dose increases,
in general, serve little
purpose.
| TABLE 4.1 — STEPS: Factors
to be Considered When Selecting a Medication for
a Patient |
- Safety
– Acute therapeutic index
– Long-term safety
– Risk of drug-drug interactions:
• Pharmacodynamically
mediated
• Pharmacokinetically
mediated
- Tolerability
– Acute
– Long-term
- Efficacy
– Overall response rate
– Unique spectrum of activity in subpopulations
– Rate of onset
– Maintenance
– Prophylactic
- Payment (ie, cost-effectiveness)
- Simplicity
– Drug administration schedule
– Ease of optimal dosing
– Need for specific clinical or laboratory monitoring
before or during treatment
|
| References: 49,
210 |
|
 |
TABLE 4.2 —
Safety and Tolerability of
TCAs versus SSRIs |
| Consideration |
TCAs |
SSRIs |
Safety
Overdose lethality risk
Alcohol potentiation |
high
high |
low
low |
Tolerability
Anticholingeric adverse events
Antihistimine adverse events
Anti-a1 adrenergic adverse
events
Serotonin adverse events |
high
high
high
low |
low
low
low
high |
|
The differences in the clinical pharmacology of TCAs versus
SSRIs will be reviewed in this chapter in terms of the STEPS
analysis developed by the author (Table 4.1).
Safety
Table 4.2 summarizes the clinically
meaningful differences between TCAs and SSRIs in terms of
safety and tolerability. Due to the fact that SSRIs do not
inhibit fast sodium channels, there is essentially no risk
of lethality, even with a substantial overdose of these medications.
In contrast, overdoses of a TCA of as little as 5 times the
daily dose can be lethal.225
This issue is important because of the delay between starting
an antidepressant of any class and achieving a meaningful
improvement in the depressive syndrome.
The risk of a suicide attempt is a serious
concern during this delayed onset interval. For many years,
TCAs have been a leading cause of death due to drug overdose
because of their serious toxicity profile, coupled with the
fact that they are given to patients at risk for making serious
suicide attempts.52,97,165
Because SSRIs avoid this problem, the prognosis of patients
suffering from this condition has significantly improved.
It used to be axiomatic to warn patients
about the dangers of drinking alcohol while taking antidepressants.
While it is still wise to advise patients against drinking
alcohol when clinically depressed, the reason is no longer
because the antidepressant will necessarily cause serious
potentiation of the central nervous system (CNS) depressant
effects of alcohol and other CNS depressants such as benzodiazepines.
Such potentiation occurs when TCAs and alcohol are taken together
due to the antihistaminic effects of TCAs, particularly tertiary
amine TCAs. Since SSRIs have been designed to avoid blocking
the histamine receptor, they do not pharmacodynamically potentiate
the effect of alcohol or other CNS depressants.76,123,155,251
Instead, they either do nothing or may mildly antagonize the
depressant effects of alcohol.76,123,155,251
This change is just one example of the reduced risk of serious
pharmacodynamic drug interactions due to
the more focused pharmacology of SSRIs. Parenthetically, fluvoxamine
and fluoxetine can pharmacokinetically potentiate the effects
of 2-keto and triazolo benzodiazepines, such as diazepam and
alprazolam respectively, by inhibiting the cytochrome P450
(CYP) enzymes responsible for their clearance (see Section
8).
The avoidance of pharmacodynamically mediated drug-drug
interactions is important because of the length of time that
patients may be on antidepressants to prevent relapse or recurrence
of depressive illness. As discussed in Section
8, antidepressants are frequently used in combination
with other drugs for a variety of reasons:
- Concomitant medical illness
- Augmentation strategies
- The addition of another drug to reduce a nuisance adverse
effect (eg, cisapride to treat nausea which can occur early
in SSRI treatment)
In addition, patients may drink alcohol socially while taking
an antidepressant and then try to drive home. Alternatively,
they may take "over-the-counter" (OTC) preparations
and have an interaction (ie, taking an OTC drug that has sedative
properties). If they are taking a tertiary amine TCA and drink
alcohol or take such an OTC product, they may experience serious
potentiation of sedative effects that may be dangerous, particularly
if they are in a situation where they need to be mentally
alert with good reaction time and coordination.
Due to the multiple pharmacodynamic effects of TCAs, there
are multiple ways that they can interact with other types
of drugs beyond simply the potentiation of CNS depressants.
Since TCAs block a1-adrenergic receptors,
they lower peripheral resistance.103
If the patient is also taking other medications that lower
blood pressure (eg, diuretics, ß-blockers), they may
experience a marked potentiation of the
orthostatic hypotension that can occur with drugs that block
a1-adrenergic receptors, that can have serious
consequences in terms of falls and resultant trauma, particularly
in the elderly.236
Since SSRIs have been also designed to avoid blocking the
a1-adrenergic receptor, they do not potentiate
the effects of concomitantly prescribed antihypertensive medications,
in contrast to TCAs. This is another example of the reduced
risk of pharmacodynamically mediated drug interactions as
a result of the more focused pharmacology of SSRIs.
Since TCAs block the muscarinic acetylcholine receptor,
they can have additive effects with other drugs that also
block this receptor or which affect gastrointestinal tract
motility through other actions. Since TCAs inhibit fast sodium
channels, they can potentiate the effects of other drugs which
affect intracardiac conduction. Such drugs include a variety
of antiarrhythmics, calcium channel blockers, and B-blockers.
SSRIs, because of their more focused pharmacology, have a
much more limited range of pharmacodynamically mediated drug-drug
interactions than do TCAs.
Tolerability
| TABLE 4.3 — Placebo-adjusted
Incidence Rate (%) of Frequent Adverse Effects on Imipramine* |
| Headache |
– 8.7 |
| Nervousness |
3.6 |
| Tremors |
10.0 |
| Insomnia |
0.4 |
| Drowsiness |
12.0 |
| Fatigue |
7.6 |
| Anorexia |
— |
| Dizziness |
22.7 |
| Vision disturbance |
5.4 |
| Palpitations |
— |
| Respiration |
– 2.3 |
| Nausea |
1.3 |
| Dyspepsia
|
— |
| Diarrhea |
– 2.7 |
| Dry mouth |
47.1 |
| Constipation |
17.4 |
| Frequent micturition |
— |
| Urinary retention |
4.0 |
| Sweating |
11.2 |
| * n = 367, placebo
= 672 |
| Placebo-adjusted
= incidence on drug minus incidence on parallel, placebo
group in a double-blind, randomly assigned clinical trial. |
| Data from reference:
211 |
Another important difference between SSRIs and TCAs is a
better overall tolerability profile in terms of a lower incidence
of both nuisance and serious adverse effects. SSRIs affect
fewer sites of action (SOAs) and hence cause fewer types of
adverse effects. Table 4.3 shows the relative incidence of
adverse effects on imipramine as a representative TCA. Imipramine
was chosen because it has historically been and still is one
of the most commonly used TCAs. Table
5.2 shows the relative incidence of the same adverse effects
on 4 of the 5 SSRIs. A comparison of these 2 tables reveals
that imipramine in comparison to the SSRIs is associated with
a considerably higher incidence of adverse effects mediated
by the blockade of specific neuroreceptors such as muscarinic
acetylcholine receptors (eg, dry mouth, constipation) and
a1-adrenergic receptors (eg, dizziness).
In contrast, SSRIs as a group have a higher incidence of adverse
effects mediated by the indirect potentiation of serotonin
via the inhibition of its uptake pump (eg, nausea). Figure
4.1 provides a visual representation of the same phenomena
using amitriptyline as another representative tertiary amine
TCA and sertraline as a representative SSRI.
While these adverse effects are less dramatic than the potentially
life-threatening toxicity problems that can occur while taking
TCAs, they can have serious consequences. The orthostatic
hypotension that can occur on TCAs may cause falls with resultant
trauma.236 The chronic
anticholinergic effects can lead the patient to discontinue
treatment during the maintenance phase of treatment and thus
increase the risk of relapse. In clinical studies, the discontinuation
of tertiary amine TCAs such as imipramine can be 3 times higher
than the discontinuation rate on an SSRI (eg,
22% versus 7% respectively).211
| FIGURE 4.1 — Comparative
Incidence of Side Effects Between Amitriptyline
and Sertraline |
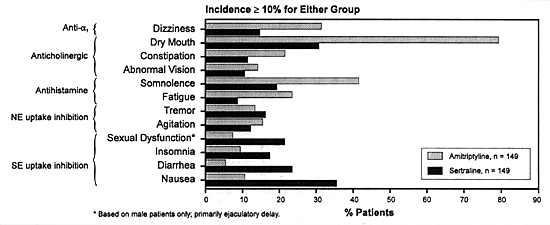 |
| Treatment-emergent side
effects (all causalities) in double-blind, placebo-controlled,
outpatient trial. |
| Reference: 237
|
|
|
 |
| TABLE 4.4 —
Response Rates in Patients With Major Depressive
Disorder by Meta-analysis |
| TCAs and SSRIs (as
a class) vs. Placebo |
Drug |
Placebo |
Difference |
n |
p
value |
| TCAs |
62.8% |
35.9% |
26.9% |
5159 |
< 10 –40 |
| SSRIs |
66.5% |
38.1% |
28.4% |
2216 |
<
10 –30 |
| |
SSRI |
TCA |
Difference |
n |
p value |
| TCAs vs. SSRIs |
77.2 |
76.9 |
0.3% |
850 |
NS |
| NS =
Not significant |
| From
reference: 135 |
|
Efficacy
While rational drug development reduces the risk of safety
and tolerability problems with SSRIs versus TCAs, it does
not reduce the relative antidepressant efficacy of the SSRIs.
As a group, they are as effective as TCAs in the treatment
of outpatients with major depression. This conclusion is supported
by a meta-analysis of the double-blind clinical trials of
TCAs and SSRIs versus placebo and the double-blind trials
directly comparing the antidepressant efficacy of SSRIs and
TCAs (Table 4.4). In this table, efficacy
is presented as response rate on each treatment after a 6-week
treatment trial. A responder is defined as a patient who experienced
at least a 50% reduction in the depressive symptomatology
as assessed by a standardized assessment
instrument, such as the Hamilton Depression Rating Scale (HDRS).
The conclusion that TCAs and SSRIs have comparable antidepressant
efficacy in this group of patients is based on the fact that
they both produce overall response rates of 60% to 65% and
that these rates are comparably superior to those achieved
in a double-blind, parallel, placebo-treated, control group.
Both the SSRIs and the TCAs produce a 25% to 30% higher response
rate than placebo. This difference between drug and placebo
is also statistically superior to a comparable degree.
Nonetheless, these results do not necessarily mean that
exactly the same patients respond to SSRIs and TCAs. Some
investigators have suggested that TCAs may work better in
patients who are hospitalized for major depression.69,70,244
That opinion is based on a few double-blind, active, controlled
studies. However, this issue remains controversial and is
not presently resolved. There are also studies showing that
up to 50% patients who fail to respond to desipramine or a
similar TCA will respond to an SSRI and vice versa.1,2,81,124,164,190,191
That finding further suggests that the spectrum of antidepressant
activity by these 2 classes of drugs is not identical. However,
considerable work needs to be done to confirm or reject these
hypotheses. Nonetheless, current data do suggest that it is
clinically reasonable to give a patient who has failed on
an SSRI a trial of a TCA or related drug as well as vice versa.
Payment
This topic is clearly important and is part of the STEPS
analysis.49 However,
a full discussion is beyond the scope of this book, given
its focus on clinical pharmacology. Nonetheless, the clinical
pharmacology of SSRIs versus TCAs must be considered when
analyzing the issue of payment or cost-effectiveness
of these different classes of antidepressants. The acquisition
cost of SSRIs is higher than that of TCAs but that is only
one part of the cost-effectiveness equation. To be meaningful,
a cost-effectiveness analysis must be much more comprehensive
and consider such factors as:
- The cost of administering the treatment
- Number of physician visits
- Ancillary tests that are needed to monitor the treatment
- The cost of treating potential adverse outcomes that
may range from nuisance side effects to serious toxicity
such as can occur with an overdose of a TCA
- The cost of an adverse outcome due to an adverse drug-drug
interaction
- The cost of effective treatment must be balanced against
the cost of not effectively treating the illness (eg, loss
productivity), including increased relapse rates in patients
who discontinue treatment before the drug has a chance to
work or who relapse because of discontinuing treatment too
early because of intolerable side effects
Simplicity
Simplicity refers to how easy it is for the physician to
prescribe the optimal dose and for the patient to take it.
One advantage shared by both TCAs and SSRIs is that they can
generally be taken once a day and be effective. Other than
this shared feature, optimal dosing with TCAs is often more
problematic than with SSRIs.
Traditionally, treatment with TCAs is begun at what is usually
a subtherapeutic dose and gradually titrated upward to an
effective antidepressant dose. This approach
is taken so that the patient can develop some tolerance to
the adverse effects caused by these drugs due to their ability
to block specific neuroreceptors as discussed earlier in this
section. In contrast, SSRIs as a class can usually be started
at the effective dose from the beginning. As discussed in
the next section, there is generally no need to titrate the
dose of the SSRIs upward in most patients.
With TCAs, the physician can use therapeutic drug monitoring
(TDM) to ensure that the patient is achieving plasma drug
concentration within a range associated with the optimal antidepressant
response with the minimum risk of adverse effects in most
patients.221 This
is an advantage from a clinical pharmacology perspective,
but is often viewed as a cumbersome disadvantage from a clinical
practice standpoint. As discussed in the next section, TDM
can also be used with SSRIs for the same purpose, although
it is rarely done.
The difference between TCAs and SSRIs with regard to TDM
is that it is a standard of care issue with TCAs, but not
with SSRIs, due to the difference in their therapeutic indexes
(ie, the difference between a dose that is therapeutic and
one that is toxic). Patients who are slow metabolizers of
TCAs can develop potentially toxic concentrations on conventional
antidepressant doses of these medications because of the multiple
SOAs affected by TCAs over their clinically relevant pha rmacological
range. If the patient slowly clears these drugs either due
to a genetically- or environmentally-induced deficiency in
the cytochrome P450 enzymes responsible for their biotransformation
and eventual elimination, they can develop concentrations
that affect fast sodium channels and, hence, delay intracardiac
conduction. Sufficient slowing can lead to conduction delays
and set the stage for potentially life-threatening cardiac
arrhythmias even though the patient is taking what is normally
a therapeutic dose. The avoidance of potentially toxic concentrations
is the primary reason for using TDM with TCAs.221
|

