|
|
Clinical Pharmacology of SSRI's
3 - Basic Neuropharmacology of SSRIs |
|
|
|
|
|
Potency and selectivity are fundamental pharmacological
concepts essential to understanding the basic neuropharmacology
and the clinical psychopharmacology of serotonin selective
reuptake inhibitors (SSRIs) including:
- Why SSRIs differ so much from the tricyclic antidepressants
(TCAs)
- Why SSRIs are so similar in terms of their psychiatric
effects
In this section, we will:
- Review results from several studies that examine the in
vitro binding affinities of SSRIs and their major metabolites
for clinically important neural receptors and uptake pumps
(ie, sites of action [SOAs])
- Contrast these binding affinities with those of clomipramine
and its major metabolite, desmethylclomipramine, to illustrate
the basic pharmacological differences between the TCAs and
the SSRIs
How In Vitro Studies Are
Done to Determine Potency
As discussed in Section 2, the
goal in developing the SSRIs is to design drugs capable of
inhibiting the neuronal uptake pump for serotonin as with
the TCAs, but at the same time, avoiding actions on other
neural mechanisms. The first step involves isolating these
neural SOAs so that the effects of drugs on them can be
studied in vitro.34,129
One approach is to isolate the neuronal uptake pump for serotonin
by homogenizing brain regions rich in serotonin terminal fields.
The homogenization process lysises the neuronal membranes
in such a way that the membrane can close back on itself to
form synaptosome preparations which retain the functional
integrity of the serotonin uptake pump. The pumps allow the
synaptosomes to concentrate serotonin by taking it up from
the fluid in which the synaptosomes are suspended. By radioactively
tagging the serotonin, the rate of uptake can measure by adding
tagged serotonin to the suspension for a specified period
of time, then centrifuging and counting radioactivity in the
synaptosomal pellet and expressing the result as the amount
of radioactivity per milligram of protein. The ability of
different drugs to slow or inhibit the pump can then be studied
by adding different concentrations of a specific drug to identical
aliquots of the same synaptosomal preparation and studying
ability of the synaptosomal preparation to take up radioactive
serotonin as a function of the concentration of the inhibitor
which has been added. All other variables, besides the amount
of inhibitor added, are kept the same among the different
aliquots.
The results from such a study are plotted in Figure 3.1
as a classic concentration-response curve in which the Y-axis
is the response (ie, effect of the drug) and the X-axis is
increasing concentration of the investigational drug (ie,
the potential inhibitor). In the case of the serotonin uptake
pump, the effect is the degree of slowing or inhibition of
the uptake of the radioactive serotonin into the synaptosomes.
This approach represents a biological assay of the effect
of the drug on its SOA rather than simply the binding affinity
of the drug for the
SOA.
| FIGURE 3.1 Generic Curve of
a Drugs Concentration-dependent Effect on Specific SOA* |
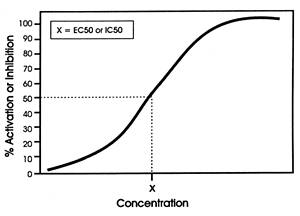 |
| * The effect (ie, activation
or inhibition) of the drug on the site of action (SOA)
is the drugs mechanism of action (MOA). |
In another version of Figure 3.1, the Y-axis can be the binding
affinity of the drug for a specific SOA (eg, the histamine1
receptor) rather than its effect on the site.66
In this case, the affinity of the drug for a receptor is measured
by its ability to displace a radioactive tagged ligand. From
a binding assay, one cannot tell whether the drug is an agonist
or an antagonist at that specific SOA; instead, only the affinity
of the drug for the receptor is determined.
In either approach, the inflection point is a reproducible
measure of the drug's affinity for the site or its effect
on the site and hence can be used for comparison purposes
across different drugs (ie, relative in vitro potency
for that SOA).
As discussed in Section 2, such
studies have been done as part of the development process
of all of the SSRIs to determine what chemical structure will:
- Convey high affinity for the serotonin uptake pump
- Slow or inhibit the pump when it bound to it
- Have low affinity for the multiple neuroreceptors
known to be responsible for many of the adverse effects
of the TCAs (eg, acetylcholine, histamine, and adrenergic
receptors)
- Not inhibit fast sodium channels which cause the
cardiotoxicity problems associated with TCAs
|
Results of In Vitro Studies
Done on the Effects of Different SSRIs on Different Biogenic
Amine Uptake Pumps
Tables 3.1, 3.2 and 3.3 show the results from three different
in vitro studies comparing the effects of four representative
TCAs and all five SSRIs on the neuronal uptake pumps for serotonin,
norepinephrine and dopamine. As can be seen, the SSRIs are
all more potent inhibitors of serotonin uptake than are the
TCAs, with the exception of clomipramine, which is less potent
than paroxetine or sertraline, approximately equal to citalopram,
and more potent than fluoxetine or
fluvoxamine.
| |
|
| TABLE 3.1
Effect of Antidepressants on Serotonin Uptake In
Vitro* |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Sertraline |
0.19 |
0.85 |
3.40 |
| Paroxetine |
0.29 |
0.44 |
0.73 |
| Clomipramine |
1.50 |
2.25 |
-- |
| Citalopram |
1.80 |
2.71 |
-- |
| Fluvoxamine |
3.80 |
3.08 |
-- |
| Fluoxetine |
6.8 |
87.0 |
93.0 |
| Imipramine |
35.00 |
31.80 |
41.00 |
| Amitriptyline |
39.00 |
67.20 |
84.00 |
| Desipramine |
200.00 |
182.00 |
180.00 |
| Relative Potency
of Antidepressant on 5-HT Uptake In Vitro |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Sertraline |
1.0 |
2.0 |
4.7 |
| Paroxetine |
1.5 |
1.0 |
1.0 |
| Clomipramine |
8.0 |
5.0 |
-- |
| Citalopram |
10.0 |
6.0 |
-- |
| Fluvoxamine |
20.0 |
7.0 |
-- |
| Fluoxetine |
36.0 |
25.0 |
19.0 |
| Imipramine |
184.0 |
95.0 |
56.0 |
| Amitriptyline |
205.0 |
153.0 |
115.0 |
| Desipramine |
1,053.0 |
414.0 |
247.0 |
* IC50 values
(nM); rat brain tissues for Studies 1 and 2 and
kinetic inhibition constant (Ki) for Study 3.
Determined by dividing the IC50 for each drug
by the IC50 for the most potent drug in each study.
The lower the number, the greater the potency. |
| References:
1129,
2250,
334 |
|
| TABLE 3.2
Effect of Antidepressants on Norepinephrine Uptake
In Vitro* |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Sertraline |
160.00 |
159.00 |
220.00 |
| Paroxetine |
81.00 |
22.20 |
33.00 |
| Clomipramine |
21.00 |
14.60 |
-- |
| Citalopram |
6,100.00 |
2,750.00 |
-- |
| Fluvoxamine |
620.00 |
299.00 |
-- |
| Fluoxetine |
370.00 |
85.30 |
143.00 |
| Imipramine |
14.00 |
12.00 |
14.00 |
| Amitriptyline |
24.00 |
14.20 |
13.90 |
| Desipramine |
0.83 |
0.65 |
0.61 |
| Relative Potency
of Antidepressant on Norepinephrine Uptake In
Vitro |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Sertraline |
193.0 |
245.0 |
360.0 |
| Paroxetine |
98.0 |
34.0 |
54.0 |
| Clomipramine |
25.0 |
22.0 |
-- |
| Citalopram |
7,349.0 |
4,231.0 |
-- |
| Fluvoxamine |
747.0 |
460.0 |
-- |
| Fluoxetine |
446.0 |
131.0 |
234.0 |
| Imipramine |
17.0 |
18.0 |
23.0 |
| Amitriptyline |
29.0 |
22.0 |
21.0 |
| Desipramine |
1.0 |
1.0 |
1.0 |
* IC50 values
(nM); rat brain tissues for Studies 1 and 2 and
kinetic inhibition constant (Ki) for Study 3.
Determined by dividing the IC50 for each drug
by the IC50 for the most potent drug in each study.
The lower the number, the greater the potency. |
| References:
1129,
2250,
334 |
|
| |
| |
| TABLE 3.3
Effect of Antidepressants on Dopamine Uptake In
Vitro* |
| Relative Potency
of Antidepressant on Dopamine Uptake In Vitro |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Sertraline |
48 |
78 |
260 |
| Paroxetine |
5,100 |
540 |
1,700 |
| Clomipramine |
4,300 |
3,810 |
-- |
| Citalopram |
40,000 |
> 10,000 |
-- |
| Fluvoxamine |
42,000 |
10,000 |
-- |
| Fluoxetine |
5,000 |
3,160 |
3,050 |
| Imipramine |
17,000 |
10,000 |
11,000 |
| Amitriptyline |
5,300 |
> 10,000 |
8,600 |
| Desipramine |
9,100 |
6,530 |
11,000 |
| Relative Potency
of Antidepressant on Dopamine Uptake In Vitro |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Sertraline |
1 |
1 |
1 |
| Paroxetine |
106 |
7 |
7 |
| Clomipramine |
90 |
49 |
-- |
| Citalopram |
830 |
> 125 |
-- |
| Fluvoxamine |
875 |
> 125 |
-- |
| Fluoxetine |
104 |
41 |
12 |
| Imipramine |
350 |
> 125 |
141 |
| Amitriptyline |
110 |
> 125 |
110 |
| Desipramine |
190 |
84 |
141 |
* IC50 values
(nM); rat brain tissues for Studies 1 and 2 and
kinetic inhibition constant (Ki) for Study 3.
Determined by dividing the IC50 for each drug
by the IC50 for the most potent drug in each study.
The lower the number, the greater the potency. |
| References:
1129,
2250,
334 |
|
The results from the three studies
also illustrate the variability that can be obtained in terms
of rank order, particularly when the drugs are relatively
close in potency. In Study 1, sertraline is approximately
twice as potent as paroxetine, whereas in the other two studies,
paroxetine is 2- to 5-times more potent than sertraline. Therefore,
the rank order shown on the bottom half of the table can change
somewhat from one study to the next.
Nonetheless, SSRIs are all substantially more potent in terms
of their affinity for the serotonin pump compared with their
affinity for or action on any other neurotransmitter pumps
or neuroreceptors. When drugs are this selective, differences
in potency after a point become clinically irrelevant since
the concentration can be adjusted to achieve inhibition of
the desired target without affecting any other target. This
fact is the essence of the concept of pharmacological selectivity
(Figure 3.2).
In Table 3.2, the results for the
same drugs from the same studies are shown with regard to
their inhibition of the norepinephrine reuptake pump. As can
readily be seen, the TCAs are substantially more potent with
regard to this action in comparison to all of the SSRIs. As
shown on the bottom half, all the SSRIs are two to three orders
of magnitude less potent than is the TCA, desipramine, in
terms of the ability to inhibit the norepinephrine uptake
pump.
In Table 3.3, results are shown for
the inhibition of the dopamine uptake pump. None of the TCAs
or the SSRIs have substantial action on this neurotransmitter
pump. Although sertraline is consistently the most potent,
it is still 100 times less potent in terms of inhibiting the
dopamine versus the serotonin uptake pump. That means the
physician would have to increase the dose (ie, the concentration)
of sertraline 100 times higher than that needed to inhibit
the serotonin uptake pump before a comparable effect would
be achieved on the dopamine uptake pump. Thus, the ratios
shown in the bottom of Table 3.3 can
be misleading if not viewed within the context of the actual
affinity of the drug for a secondary SOA relative to its affinity
for its primary SOA and relative to the clinically relevant
concentration needed to produce the desired clinical effect.
Recall that citalopram and fluoxetine are marketed as racemates
(see Section 2). The values shown
in the above tables for uptake inhibition are
for the racemates of these two SSRIs. Table
3.4 shows the value for the individual enantiomers of
each of these SSRIs and their major metabolite.
| FIGURE 3.2 Selectivity Ratios
for a Series of Uptake Inhibitors Measured In Vitro |
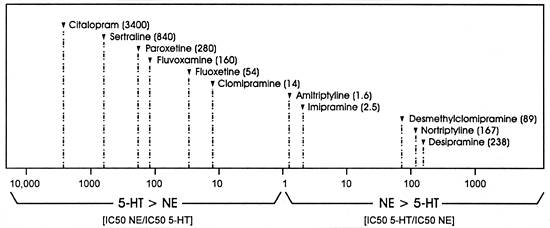 |
| Data from reference: 129 |
The Concept of Selectivity as Related
to Effects on Different Biogenic Amine Uptake Pumps
The concept of selectivity is further illustrated in Table
3.5. In this table, the affinity of a specific drug for
the norepinephrine uptake pump is divided by its affinity
for the serotonin uptake pump. As seen in Tables 3.1,
3.2 and 3.3, the
more potent a drug, the smaller the concentration needed to
affect or bind to an SOA. Thus, the less potent effect is
a larger number (ie, more concentration is needed to produce
the same degree of effect), and the more potent effect is
the smaller number. In Table 3.5, the
ratio is appreciably greater than "1" for all SSRIs,
whereas the ratio for all TCAs, except clomipramine, is considerably
smaller than "1." SSRIs are considerably more potent
at inhibiting the serotonin uptake pump than the norepinephrine
uptake pump, whereas the opposite is true for the TCAs, with
the exception of clomipramine.
The more the ratio diverges from "1" in either
direction, the more selective the drug is for one pump over
the other. For example, all SSRIs, with the exception of fluoxetine,
are more than 100 times more potent at inhibiting the serotonin
versus the norepinephrine uptake pump, whereas the converse
is true for the TCA, desipramine. A concentration of any SSRI
that will produce substantial inhibition of the serotonin
uptake pump will produce no physiologically meaningful inhibition
of the norepinephrine uptake pump. The converse will be true
for TCAs such as desipramine. Clinically, such selectivity
ratios translate into being able to produce all the physiological
effects mediated by inhibiting one pump
without causing any effects that will be produced by inhibiting
the other uptake pump.
Figure 3.2 graphically illustrates
the same point. In this figure, a value of "1" means
that the drug will inhibit both uptake pumps at the same concentration
(ie, no selectivity with regard to effect on these two SOAs).
For illustration purposes, the ratio of the right side of
the figure is the potency for inhibiting the uptake of serotonin
divided by the potency for inhibiting the uptake for norepinephrine,
while the inverse is demonstrated on the left side of the
figure. This approach is taken so that the ratios will become
larger in either direction and hence may be intuitively simpler
to understand. In this figure, desipramine is 238 times more
potent at inhibiting the norepinephrine uptake pump versus
the serotonin uptake pump, whereas all the SSRIs, with the
exception of fluoxetine, are over 100 times more potent at
inhibiting the serotonin versus the norepinephrine uptake
pump.
Affecting any SOA can cause adverse as well as beneficial
effects. The physiological responses mediated by activation
or inhibition of these and other SOAs are listed in Table
3.6. The goal of rational drug development is to be able
to produce drugs that affect the SOA necessary to mediate
the desired effect without affecting an SOA that is not critical
to producing the desired effect. Affecting unnecessary SOAs
can lead to unnecessary adverse effects and an increased potential
for causing pharmacodynamic drug-drug interactions.
Potency Relates to Concentration,
Not Dose
There is a frequent misconception that potency refers to
the dose of a drug needed to produce an effect. That is wrong.
Instead, it refers to the concentration
of a drug needed to produce an effect. Two drugs may be able
to produce exactly the same effect, but the concentration
needed of each drug may be quite different. The drug that
requires a lower concentration to achieve the same magnitude
of effect is the more potent drug regardless of the dose needed
to achieve that concentration.
Although dose is sometimes used as the reference point,
it is usually because the concentration has not been measured
or the author may not be aware of how misleading a dose comparison
can be. The concentration achieved by a given dose of a drug
is dependent on the bioavailability and elimination rate of
the drug. A drug that has lower bioavailability and/or a faster
clearance will require a higher dose to produce the same concentration
as a drug which has a greater bioavailability or a slower
clearance. The critical issue for
the SOA is not what dose is taken, but what concentration
is achieved at the SOA.
| TABLE 3.4
Relative Potency of the Enantiomers of Citalopram,
Fluoxetine and Their Metabolites for Inhibiting
the Uptake Pumps for Different Biogenic Amine Neurotransmitters |
| Drug |
5-HT |
NE |
DA |
| Racemic citalopram |
1.8 |
6100 |
40,000 |
| S-citalopram |
1.5 |
2500 |
65,000 |
| R-citalopram |
250 |
6900 |
54,000 |
| S/R
ratio* for citalopram = 0.562 |
| Racemic desmethylcitalopram |
14 |
740 |
28,000 |
| S-desmethylcitalopram |
10 |
1500 |
34,000 |
| R-desmethylcitalopram |
65 |
500 |
25,000 |
| S/R
ratio* for desmethylcitalopram = 0.692 |
| Drug |
5-HT |
NE |
DA |
| Racemic fluoxetine |
20 |
1,230 |
2,880 |
| S-fluoxetine |
22 |
2,040 |
2,510 |
| R-fluoxetine |
35 |
562 |
2,820 |
| S/R
ratio* for fluoxetine = 2.24 |
| Racemic norfluoxetine |
45 |
2,400 |
2,190 |
| S-norfluoxetine |
14 |
4,270 |
2,750 |
| R-norfluoxetine |
309 |
3,720 |
2,140 |
| S/R
ratio* for norfluoxetine = 2.24 |
| * S/R ratio
= ratio of plasma levels of the two enantiomers
under steady-state condition when the racemic mixture
is being taken. |
| References:
1130,
2242,
3290,
4272 |
|
| |
 |
| TABLE 3.5
In Vitro Selectivity Ratio* for Different SSRIs
and Selected TCAs |
| Drug |
Study 11 |
Study 22 |
Study 33 |
| Paroxetine |
280.0 |
50.0 |
64.0 |
| Sertraline |
840.0 |
187.0 |
45.0 |
| Clomipramine |
14.0 |
6.5 |
-- |
| Citalopram |
3400.0 |
1015.0 |
-- |
| Fluvoxamine |
160.0 |
97.0 |
-- |
| Fluoxetine |
54.0 |
8.0 |
10.0 |
| Imipramine |
0.4 |
0.3 |
0.3 |
| Amitriptyline |
0.6 |
0.2 |
0.2 |
| Desipramine |
0.004 |
0.004 |
0.003 |
| * (IC50 NE
uptake/IC50 5-HT uptake) |
| References:
1129,
2250,
334 |
|
| |
 |
| TABLE
3.6 Pharmacologic Properties of Antidepressants
and Possible Clinical Consequences |
| Property |
Consequences |
| Blockade of histamine (H-1and
H-2) receptors |
Sedation, drowsiness;
potentiation of central depressant drugs; weight
gain |
| Blockade of muscarinic
acetylcholine receptors |
Dry mouth, blurred vision,
sinus tachycardia, constipation, urinary retention,
memory impairment |
| Blockade of
norepinephrine uptake at nerve endings |
Antidepressant
efficacy (?); tremors, jitteriness; tachycardia;
diaphoresis; blockade of the antihypertensive effects
of guanethidine; augmentation of pressor effects
of sympathomimetic amines; erectile and ejaculatory
dysfunction |
| Blockade of serotonin uptake
at nerve endings |
Antidepressant efficacy
(?); sexual dysfunction; nausea, vomiting, diarrhea;
anorexia; increase or decrease in anxiety (dose-dependent);
asthenia (tiredness); insomnia; extrapyramidal side
effects; interactions with L-tryptophan, monoamine
oxidase inhibitors, fenfluramine, and occasionally
lithium |
| Blockade of serotonin-2
(5-HT2) receptors |
Antidepressant efficacy
(?), ejaculatory dysfunction, hypotension, alleviation
of migraine headaches, decrease in anxiety (?),
decrease motor restlessness (?) |
| Blockade of a1-adrenergic
receptors |
Postural hypotension, dizziness
which predisposes to falls possibly resulting in
broken bones and subdural hematomas, potentiation
of antihypertensive drugs |
| Blockade of a2-adrenergic
receptors |
Priapism; blockade of the
antihypertensive effects of clonidine, a-methyldopa,
guanabenz, guanfacine |
| Blockade of fast sodium
channels |
Slow repolarization, delay
intracardiac conduction, reduce some arrhythmias
at low concentrations, cause arrhythmias, seizures
at high concentrations |
| References:
188, 225,
239 |
|
| |
 |
| TABLE 3.7
Relationship Between Dose, Plasma Level, Potency
and Serotonin (5-HT) Uptake |
| SSRI |
Usually Effective Dose (mg/day)* |
Plasma
Level |
In Vitro Potency IC506 |
Inhibition of 5-HT Uptake Pump (%) |
| Citalopram |
40 |
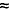 85 ng/ml (260 nM)1
85 ng/ml (260 nM)1 |
1.8 (14) |
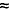 60%7
60%7 |
| Fluoxetine |
20 |
 200 ng/ml (660 nM)2
200 ng/ml (660 nM)2 |
6.8 (3.8) |
 80%8
80%8 |
| Fluvoxamine |
150 |
 100 ng/ml (300 nM)3
100 ng/ml (300 nM)3 |
3.8 |
 70%9
70%9 |
| Paroxetine |
20 |
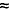 40 ng/ml (130 nM)4
40 ng/ml (130 nM)4 |
0.29 |
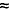 80%10
80%10 |
| Sertraline |
50 |
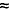 25 ng/ml (65 nM)5
25 ng/ml (65 nM)5 |
0.19 (NA)§ |
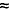 80%11
80%11 |
*
Refer to Section 5 for this discussion.
Plasma level for fluoxetine represents
total of fluoxetine plus norfluoxetine given comparable
effect of each on 5-HT uptake pump; parent SSRI
alone shown for all others. Also, plasma levels
are a total of both enantiomers for citalopram and
fluoxetine.
Value for parent drug and value for respective
major metabolite are in parentheses.
§ Not available from this study. Refer to
Table 3.8. |
| References:
131, 93,
146, 179,
197, 242;
286, 159,
219, 232;
390, 182;
421, 122,
166, 167,
252; 55,
219, 243;
6129;
731; 8159;
9292;
10170;
11223 |
|
Table 3.7 illustrates how misleading
dose can be, using SSRIs as examples of this basic pharmacological
principle. This table shows:
- Usually effective dose of each SSRI
- Usual concentration of each drug at its usually effective
dose achieved
- In vitro potency for inhibiting the serotonin
uptake pump
- In vivo degree of serotonin uptake inhibition achieved
by each drug at its usually effective, antidepressant dose
in man using the platelet as a surrogate for the serotonin
neurons since platelets, like serotonin neurons, have a
serotonin uptake pump
As can be seen, there is little correlation between the dose
of the drug and the plasma concentration achieved. For example,
the combined plasma concentration of fluoxetine and its active
metabolite, norfluoxetine, is 10 times higher
than the concentration of sertraline even though the dose
of fluoxetine is 2.5 times less than the dose of sertraline.
If the comparison was made on the basis of dose, fluoxetine
were erroneously appear to be more potent than sertraline
as an inhibitor of serotonin uptake. While the dose of SSRIs
does not correlate with their in vitro potency, there
is a clear correlation between the in vitro potency
of the drug and the plasma level of each drug needed to produce
relatively comparable serotonin uptake inhibition (ie, lower
plasma concentrations of the more potent SSRIs [eg, paroxetine,
sertraline] are needed in comparison to higher concentrations
of the less potent SSRIs [eg, citalopram, fluoxetine, fluvoxamine]).
The results in Table 3.7 are of clinical
and research interest. Each SSRI, at the dose found to be
its usually effective, minimum dose based on double-blind,
placebo-controlled studies, produces approximately 70% to
80% inhibition of the serotonin uptake pump using the platelet
as a surrogate marker. This finding is consistent with the
concept that the inhibition of this pump is relevant to the
antidepressant efficacy of these drugs and suggests that approximately
70% to 80% inhibition of this pump is usually necessary to
produce an antidepressant effect. Higher doses of these drugs
do not produce a greater antidepressant response on average
(ie, a flat dose-response curve for antidepressant efficacy),
but do increase the incidence and severity of adverse effects
mediated by excessive serotonin uptake inhibition (eg, agitation,
loose stools, nausea). (For more details on this issue, refer
to Figures 5.1 and 5.2
later in this book.) These two observations, coupled with
the results shown in Table 3.7, indicate
that inhibition of the serotonin uptake pump by substantially
more than 80% produces a greater increase in adverse effects
than an increase in antidepressant efficacy
and is one reason to avoid the temptation to use a dose higher
than the usually effective dose before it has been given an
adequate trial (ie, approximately 4 weeks).
Obviously, the results in Table 3.7
pertain to the average patient. A patient who has a rapid
clearance of the drug may need a higher than average dose
to achieve an effective concentration, whereas a patient who
has a slow clearance may do better in terms of the ratio of
efficacy-to-adverse effects on a dose lower than usually effective,
minimum dose. (For more details on this issue, refer to the
Therapeutic Drug Monitoring discussion in Section
5.)
The Concept of Selectivity as Related
to Effects on Different Neuroreceptors
The goal of development of SSRIs is not only to avoid affecting
the norepinephrine and dopamine uptake pumps, but a variety
of neuroreceptors (in contrast to the TCAs). Figure 3.3 illustrates
how well that goal is accomplished using clomipramine as the
reference TCA. Shown in this figure are the binding affinities
of 7 different chemical agents (ie, clomipramine and its primary
metabolite, desmethylclomipramine, and all SSRIs) for 5 clinically
important neuroreceptors as well as the 3 biogenic amine uptake
pumps. The X-axis is nanomolar concentration on an algorithmic
scale so that each vertical line represents an increase in
concentration of ten times the previous one. The further the
distance between the drug's affinity for one SOA and the next,
the greater its selectivity for affecting that target without
affecting the next potential target.
| FIGURE 3.3 In Vitro Profile
of Antidepressants |
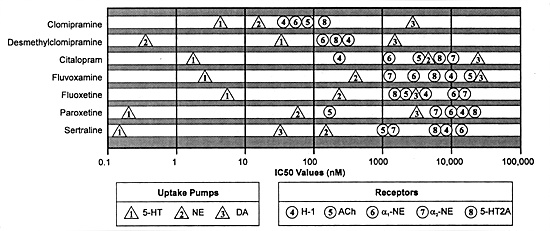 |
| Adapted from reference: 129 |
Clomipramine differs from the other tertiary amine TCAs
(eg, amitriptyline, doxepin, imipramine) in that its most
potent action is on a site believed to mediate efficacy
in major depression and also obsessive-compulsive disorders
(ie, the serotonin uptake pump). In contrast, the other tertiary
amine TCAs block the histamine receptor as their most potent
action, which is why their most potent effect is sedation
and why they can potentiate the effect of other sedative agents,
including alcohol.76,123,155,188,211,251
Clomipramine, like the other tertiary amine TCAs, has little
separation between potency for effects on multiple SOAs such
as the norepinephrine uptake pump, the histamine-1, a-1 adrenergic,
acetylcholine, 5-HT2A neuroreceptors and the dopamine uptake
pump (Figure 3.3). The widest gap for clomipramine is approximately
300 times for the inhibition of the serotonin versus the dopamine
uptake pump. That difference is such that clomipramine is
unlikely to produce meaningful effects on the dopamine uptake
pump at doses which substantially inhibit the serotonin uptake
pump. In contrast, the difference between its affinity for
the serotonin uptake pump and the other neural SOAs (eg, the
norepinephrine uptake pump and various neuroreceptors) is
10-fold or less. That difference is small enough that effects
on these sites may occur under clinically relevant conditions
and thus can contribute to the clinical pharmacology of the
drug. If clinical effects mediated by the drug's action on
these sites are unnecessary for the desired clinical effects,
these effects will be termed "side-effects" and
may range from being a nuisance to treatment-limiting problems
to serious adverse effects.
Although not shown in Figure 3.3, clomipramine, like the
other TCAs, is also capable of inhibiting fast sodium channels.57
The potency of the drug for this action is such that it occurs
to a clinically meaningful extent in the healthy individual
only at concentrations above its therapeutic range for antidepressant
efficacy and the probable reason for its dose- and, hence,
concentration-dependent seizure risk and cardiac arrhythmia
risk.59 However,
concentrations can occur in individuals
who take an overdose of the drug or who are slow metabolizers
and develop high concentrations on what are usually therapeutic
doses.221,225
Slow metabolizers are typically deficient in the cytochrome
P450 enzyme, CYP 2D6, either because of genetics or because
they are on a concomitant drug that substantially inhibits
this enzyme (eg, fluoxetine or paroxetine).45,213
Inhibition of fast sodium channels produces stabilization
of electrically excitable membranes and clinically results
in the potentially life-threatening adverse effects that TCAs
can have on the heart (eg, conduction disturbances) and the
brain (eg, seizures).33,222,225
Thus, effects of TCAs on this SOA cause their narrow therapeutic
index.
Figure 3.3 illustrates another complicating
feature of the pharmacology of clomipramine and the other
tertiary amine TCAs. They are demethylated in the body to
secondary amine TCAs which have a pharmacological profile
different from that of the parent drug. In the case of clomipramine,
this metabolite is desmethylclomipramine. Its binding affinity
for the same SOAs is shown in Figure 3.3
in the bar below that for the parent drug. As can be seen,
this metabolite, like all secondary amine TCAs, is considerably
more potent than the parent drug as an inhibitor of the norepinephrine
uptake pump and less potent as an inhibitor of the serotonin
uptake pump.34 The
conversion of clomipramine to desmethylclomipramine is mediated
by at least two CYP enzymes, CYP 1A2 and 3A3/4,43,44
and possibly 2C19.168
Activity of these 2 enzymes can vary substantially among individuals
and even within the same person because these enzymes can
be induced and inhibited by environmental factors, including
concomitant medications taken by the individual (discussed
further in Sections 7 and 8).
If inhibition of the serotonin uptake pump is critical to
the desired clinical effect of clomipramine (eg, efficacy
in some forms of major depression and in obsessive-compulsive
disorder), then a patient may fail to respond because s/he
develops higher levels of the metabolite as opposed to the
parent drug. Conceivably, a patient who had responded might
lose efficacy if exposed to an environmental agent capable
of inducing CYP 1A2 or 3A3/4 after being stabilized on what
had previously been an optimal dose of clomipramine. While
the physician can increase the dose of clomipramine sufficiently
to achieve high enough levels of the parent drug to produce
the necessary inhibition of the serotonin uptake pump, the
dose may have to be so high that effects of the metabolite
on other SOAs can become clinically meaningful, causing nuisance
and/or serious adverse effects. Thus, Figure
3.3 illustrates the potential problems inherent in having
an active metabolite with a pharmacological profile which
is meaningfully different from the parent compound.
Figure 3.3 also shows the binding
affinities for all of the SSRIs. The most potent action of
each SSRI is the inhibition of the serotonin uptake pump.
Each has a substantially higher affinity for this SOA than
for any other site shown. Stated in another way, the SSRIs
as a group show a clinically meaningful separation or selectivity
for the serotonin uptake pump versus any of the other SOAs
shown in Figure 3.3.
However, Figure 3.3 does not show
the affinity of these drugs for various CYP enzymes. In the
case of these enzymes, some of the SSRIs produce meaningful
effects at the same concentration that they affect the serotonin
uptake pump and, thus, do not show "selectivity"
in terms of distinguishing between the serotonin uptake pump
and such CYP enzymes; but instead, they can produce effects
on both of these sites under clinically relevant conditions
(discussed further in Sections 7 and 8).
What About the
Effects of SSRI Metabolites?
Given the discussion of clomipramine and desmethylclomipramine,
it is important to know whether SSRIs have active metabolites
with a substantially different pharmacological profile than
the parent drug. Table 3.8 shows the results of two in
vitro studies examining the effects of some of the SSRIs
and their primary metabolites. The results for clomipramine
and desmethylclomipramine, illustrated in Figure
3.3, are shown for comparison purposes. There are two
entries for fluoxetine and norfluoxetine because they were
examined in both in vitro studies.
As can be seen in Table 3.8, the metabolites of citalopram,
fluoxetine and sertraline have the same rank order of binding
affinity for these various SOAs as their respective parent
SSRI. Metabolites of fluvoxamine and paroxetine were not available
for testing in these studies, but they are reported to not
have metabolites with clinically meaningful affinity for any
of these SOAs.141,198
The metabolites of citalopram and sertraline are more than
10 times less potent than the parent drug for inhibiting the
serotonin uptake pump (Table 3.8). In the case of sertraline,
its metabolite is 25 times less potent than the parent drug.
Since this metabolite occurs in concentrations only 1.5 times
higher than the parent drug under clinically relevant conditions,219
this metabolite will be expected to contribute negligibly
(ie, approximately 6%) to the overall clinical pharmacology
of this drug mediated by inhibition of this SOA.
| TABLE 3.8 Effect
of Uptake Inhibitors and Their Metabolites In Vitro |
| |
5-HT
uptake |
NA
uptake |
DA
uptake |
D-2 |
5-HT2 |
a1 |
H-1 |
ACh |
| Clomipramine1 |
1.5 |
21.0 |
4,300 |
430 |
120 |
60 |
54 |
67 |
| Desmethylclomipramine1 |
40.0 |
0.45 |
2,100 |
1,200 |
340 |
190 |
450 |
92 |
| Citalopram1 |
1.8 |
6,100.0 |
40,000 |
33,000 |
9,200 |
1,600 |
350 |
5,600 |
| Desmethylcitalopram1 |
14.0 |
740.0 |
28,000 |
53,000 |
19,000 |
1,500 |
1,700 |
14,000 |
| Didesmethylcitalopram1 |
22.0 |
1,400.0 |
11,000 |
24,000 |
16,000 |
3,400 |
11,000 |
23,000 |
| Fluoxetine1 |
6.8 |
370.0 |
5,000 |
32,000 |
2,600 |
14,000 |
3,200 |
3,100 |
| Norfluoxetine1 |
3.8 |
580.0 |
4,300 |
13,000 |
2,500 |
15,000 |
11,000 |
3,400 |
| Fluoxetine2 |
14.0 |
143.0 |
3,050 |
12,000 |
280 |
3,800 |
5,400 |
590 |
| Norfluoxetine2 |
25.0 |
416.0 |
1,100 |
16,000 |
600 |
3,900 |
11,000 |
810 |
| Sertraline2 |
3.4 |
220.0 |
260 |
11,000 |
9,900 |
380 |
24,000 |
630 |
| Desmethylsertraline2 |
76.0 |
420.0 |
440 |
11,000 |
4,800 |
1,200 |
9,000 |
1,430 |
| Note the effects
of fluoxetine and norfluoxetine were measured in two different
sets of studies. Data from reference 129
are in terms of inhibition concentration, 50% maximum
effect (IC50), whereas data from references 34
and 66 are in terms of kinetic
inhibition constant (Ki) for the uptake pumps
and kinetic dissociation constant (Kd) for
the receptors. |
| References: 1129,
234, 66 |
The reverse is true for fluoxetine. In some in vitro studies,
its primary metabolite, norfluoxetine, has been found to be
somewhat more potent than the parent drug at inhibiting the
serotonin uptake pump (Table 3.8). Moreover,
norfluoxetine levels can be twice the levels of the parent
drug and persist for a substantially longer interval after
discontinuation due to its slower clearance (ie, longer half-life).142,219,232
Given its affinity for the serotonin pump and its higher,
longer-lived levels, norfluoxetine is an important metabolite
with regard to clinical effects mediated by the inhibition
of the serotonin uptake pump.
There is an important caveat to this discussion. When the
statement is made that a drug does not have an "active
metabolite," several questions should be asked:
- How well has the metabolism of the drug been clarified?
- Can there be clinically meaningful metabolites that have
not been studied or have not yet been identified?
- What does "active" mean or, in other words,
what activity has been studied?
With the exception of fluoxetine, none of the SSRIs have
metabolites with clinically relevant effects on any of the
neural sites shown in Table 3.8. However, every SSRI that
has been studied has metabolites with approximately the same
activity as the parent drug for the inhibition of specific
CYP enzymes (for details, see Table
8.7). Hence, these metabolites are "active"
with regard to inhibiting these enzymes and contributing to
the effects mediated by this action (eg, the slowing of the
clearance of drugs metabolized by these specific enzymes).
The magnitude of the contribution by the metabolite relative
to the parent drug is a function of their relative potency
for the specific mechanism of action (MOA) of interest and
their relative concentrations at the relevant SOA under clinically
relevant dosing conditions. For example, norfluoxetine is
almost 10 times more potent
than the parent drug at inhibiting the CYP enzyme, 3A3/4 (Table
8.7).
| TABLE 3.9
Effect of Metabolism on the Central MOA and Half-lives
of Some SSRIs |
| Drug |
5-HT
uptake*1 |
NE uptake1 |
Half-lives*2 |
Consequence |
| Clomipramine |
1.5 |
2.1 |
19 to 37 hrs2 |
Loss
of selectivity |
| Desmethylclomipramine |
40.0 |
0.45 |
54 to 77 hrs2 |
| Fluoxetine |
6.8 |
370.0 |
2 to 4 days2,3 |
Increased
duration of action |
| Norfluoxetine |
3.8 |
580.0 |
7 to 15 days2,3 |
| Citalopram |
1.8 |
6100.0 |
1.5 days4 |
No
change in selectivity or duration of action; no clinically
active metabolites in terms of serotonin uptake inhibition |
| Fluvoxamine |
3.8 |
620.0 |
0.5 to 1 day5 |
| Paroxetine |
0.29 |
81.0 |
1 day (at 20 mg/d)2 |
| Sertraline |
0.19 |
160.0 |
1 day2 |
* Half-live
is a major determinant of the duration of action of a
drug.
Metabolites of citalopram and sertraline are
substantially weaker inhibitors of serotonin uptake than
the parent drug. These metabolites also occur in concentrations
either about the same as the parent drug or less. Hence,
they do not contribute in a meaningful way to the effect
of the drug via this mechanism of action. However, the
metabolites of several SSRIs are as potent or more potent
as the parent drug at inhibiting specific CYP enzymes
and thus contribute to this effect (Table 8.7). |
| References: 1129,
2213, 3108,
4173, 573 |
Clinical Relevance
of Active Metabolites
The clinical implications of the differential effects of
the metabolites of clomipramine and the SSRIs on neural SOAs
are summarized in Table 3.9. In the case of clomipramine,
its metabolite can cause a loss of selectivity in terms of
effects mediated by the inhibition of the serotonin versus
the norepinephrine uptake pump. In the case of fluoxetine,
its primary metabolite has the same pharmacological profile
as the parent drug. In fact, norfluoxetine, relative to fluoxetine:
- Is a comparable or even more potent inhibitor of the serotonin
uptake pump and CYP 2D6 (Table
8.7)
- Is a more potent inhibitor of CYP 3A3/4 (Table
8.7)
- Is generally present at higher levels142,219,232
- Persists for a substantially longer period of time after
fluoxetine administration has been stopped112,200,219
For these reasons, this metabolite is clinically important
in terms of increasing the magnitude and the duration of clinical
effects mediated by the inhibition of the serotonin uptake
pump and for effects (eg, pharmacokinetic drug interactions)
mediated by the inhibition of one or more CYP enzymes.
With regard to the other SSRIs, they do not have metabolites
with sufficient activity at any known neural SOAs to alter
or contribute to the magnitude or duration of any psychiatric
effects produced by the parent drug. However, the primary
metabolites of citalopram, paroxetine and sertraline, like
fluoxetine, have similar potency to their respective parent
drug in terms of the effects on specific CYP enzymes (for
details, see Section 8). The primary
metabolites of fluvoxamine have not been
adequately studied to comment about this SSRI in this respect.
Conclusion
Understanding the rational development strategy that have
been used to produce the SSRIs lays the foundation for understanding
their basic neuropharmacology and why it differs from TCAs.
This knowledge also explains why the SSRIs are alike in so
many ways and also why the differences in their pharmacokinetics
and effects on CYP enzymes have become distinguishing characteristics
among these drugs.
|

